Servicios Personalizados
Articulo
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. vol.40 no.3 Bahía Blanca jul. 2010
Isomerization of fatty acids in sunflower oil during heat treatment
L.E. Mateos, G.M. Tonetto, G.H. Crapiste
PLAPIQUI (Universidad Nacional del Sur-CONICET), La Carrindanga Km 7, C.C. 7717,
8000 Bahía Blanca, Argentina, Fax: (54-291)4861600.
lmateos@plapiqui.edu.ar
Abstract — Pilot-scale treatments of sunflower oil similar to deodorization were carried out by applying steam stripping at different temperatures ranging from 179 to 282°C for 5 h. Samples were taken every half hour, and fatty acid composition was determined by gas chromatography.
The results showed an increase in the relative percentage of trans linoleic acid with an increase in either time or temperature. The formation of trans linoleic acid isomers followed a zero-order reaction and the kinetic constants varied according to the Arrhenius' law. The activation energies for the formation of the acids C18:2ct, C18:2tc and C18:2tt were calculated as 147.4, 147.8 and 146.6 kJ/mol, respectively.
Keywords — Deodorization. Sunflower Oil. Trans isomer Formation. Zero-Order Reaction. Linoleic Acid.
I. INTRODUCTION
Deodorization is the last stage in the refined process of vegetable oils, designed to eliminate the free fatty acids and volatile products that confer undesirable flavors and scents to the edible oils. Deodorization is feasible because of the great differences in volatility between the triglycerides present in the neutralized and blanching oils, practically non-volatile, and the substances that need to be eliminated. The deodorization is a steam-distillation process carried out at relatively high temperature (200-270°C) and low pressure (1-8 mm Hg) (Cook, 2002).
The introduction of an inert gas, or stripping steam, increases vaporization and further reduces the temperature needed for distillation. The application of reduced pressure during the operation protects the hot oil from atmospheric oxidation, prevents undue hydrolysis of the oil by water, and greatly reduces the quantity of steam needed (Norris, 1985).
During the process, other important components of the oils, such as tocopherols, tocotrienols, sterols and fatty esters, are also partially eliminated. In addition, the double bonds may isomerize from cis to trans form (Norris, 1985). As it is known, the unsaturated fatty acids can present different geometric configurations, though in vegetable oils they are naturally in the cis form. During deodorization, due to effects of temperature and time of processing, variable quantities of trans fatty acids (TFA) are formed.
TFA have been reported to be unfavorable for human diet due to their negative health effects. It has been demonstrated that the percentage of TFA contained in the diet is strongly correlated with coronary heart diseases (Mozaffarian et al., 2004; Han et al., 2002).
Deodorization is the main step of oil refining that contributes to increase the TFA content. In European countries, the quality parameters for refined edible oils include low TFA levels (Aro et al., 1998). The description "Low trans fatty acid content" implies that the product must contain less than 1% trans acid, with a normally accepted maximum of 0.8% in the finished product (Cook, 2002). In 2003, the Food and Drug Administration (FDA) ruled that the amount of TFA in a food item must be stated on the label after January 1, 2006; food items could be labeled 0% trans if they contain less than 0.5 g/serving (FDA, 2003). Although the oil is not hydrogenated, it even must be deodorized, and thus the effect of the thermally induced cis-trans isomerization becomes important. For this reason, although the formation of geometrical isomers during deodorization is known since 1974 (Ackman et al., 1974), it is still a subject of study.
The aim of the present work is to explore the effects of temperature and time on the concentration of TFA during the heat treatment of sunflower oil.
II. METHODS
A. Materials
Commercial refined sunflower oil was used in all the experiments (Natura, 5L bottles). The fatty acid composition of the oil is given in Table 1. The starting sunflower oil contained a small amount of trans isomers of oleic acid (0.22%) and a larger quantity of trans linoleic acid isomers (0,05% of 18:2tt, 0,49% of 18:2tc, and 0,37% of 18:2ct).
Table 1. Fatty acid composition (results are expressed as weight percentage total FAME) of sunflower oil before and after processing. 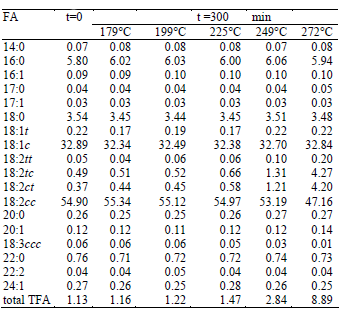
B. Pilot-scale operation
The system was composed for Parr reactor 4841 with a total capacity of 2 L, with external electric jacket, and equipped with agitator and temperature control. The striping gas was steam provided by a boiler, with a flow of 0.01 kg/min. The steam was injected into the oil through small holes arranged in the sparger system at the bottom of the reactor. Samples were collected every half hour, and then the hot samples were quickly cooled and kept under nitrogen at -20°C in dark brown glass bottles. The pressure in the equipment during operation was atmospheric because previous studies show that pressure has unimportant effects over TFA formation (Ceriani and Meirelles, 2007).
Heat treatments of sunflower oil were carried out for 5 h at 179, 199, 225, 249, and 272°C. About 60 min was needed to reach final temperature in all the experiments. The residence time was started when the test temperature was reached.
C. Analysis of fatty acid composition
Fatty acid methyl esters (FAMEs) were prepared acording to IUPAC (1992) standard method 2.301: 10 mL of a methanolic NaOH solution (0.13 M) was added to 0.15-0.20 g of oil. The mixture was boiled under reflux for 15 min. After cooling, phenolphthalein and 3 mL of 5% H2SO4/MeOH solution were added. The sample was heated again under the same conditions for 10 min. After cooling, 10 mL of saturated NaCl solution were added to dilute the methanolic solution. FAMEs were extracted with 1 mL of hexane.
Analysis of the FAMEs was made by capillary gas chromatography following procedures established by norm AOCS Ce 1c-89, using an Agilent 4890D chromatograph, equipped with a flame ionization detector (FID) and a split injector. A 100-m long SUPELCO 2560 capillary column, with a nominal diameter of 0.25 mm and a nominal film thickness of 0.20 µm, was used for the separation of the different compounds present in the samples. Hydrogen was used as carrier gas at a pressure of 18psi. Detector and injector temperature were held at 250°C and 225°C respectively. The oven tem perature was increased at a rate of 1°C/min from 130°C to 210°C, and it was held at 210°C for 20 min. FAMEs peaks were identified by comparison with the retention time of the respective standard (37 component FAME mix, Supelco). The 18:2tt, 18:2tc and 18:2ct trans isomers were clearly identified in the oil samples as it is showed in Fig. 1 (using a linoleic acid methyl ester, cis/trans-isomers mix, Sigma).
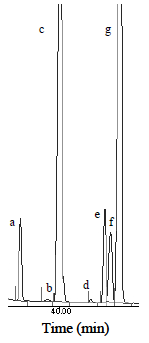
Figure 1. Partial chromatogram of fatty acid methyl esters of sunflower oil. SP-2560 capillary column, 100 m x 0.25 mm, 0.20 µm; 130°C to 210°C at 1°C/min (hold 20 mi). References: a: C18:0, b: C18:1t, c: C18:1c, d: C18:2tt, e: C18:2ct, f: C18:2tc, g C18:2cc.
D. Statistical analyses
To study the effects of the different factors and to test the statistical significance, the Analysis of Variance (ANOVA) was performed on the analytical data con cerning the total trans C18:2.
III. RESULTS AND DISCUSSION
Table 1 shows the fatty acid composition of sunflower oil before and after the heat treatment at different temperatures for 300 min. The total TFA content in the oil samples varied from to 1.13 to 8.89%. The most significant increase of the generated trans isomers is for the C18:2, since the C18:1c is little or non-reactive (Medina-Juárez et al., 2000) and the C18:3 is in very low quantity in sunflower oil.
When the C18:2 trans isomers were individually analyzed, it was found that the formation of C18:2tt is lower than the formation of C18:2tc and C18:2ct (0.20 versus 4.27 and 4.20 respectively).
Figure 2 shows the increase of the total 18:2 trans isomers (C18:2tt + C18:2tc + C18:2ct) versus time for the five operation temperatures. Values at time zero correspond to the oil before treatment. The more significant changes were observed when the highest temperature and time were applied (5 h and 272°C) with 8.67% of TFA content.
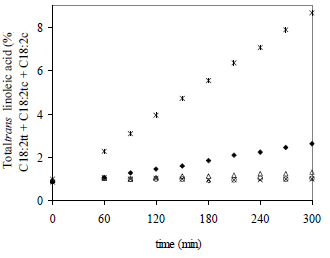
Figure 2. Total trans linoleic acid (C18:2tt + C18:2tc + C18:2ct) as a function of processing time, at different temperatures of deodorization. References: * 272°C,  249°C, Δ 225°C, o 199°C, x 179°C.
249°C, Δ 225°C, o 199°C, x 179°C.
In all experiments tested, the evolution of concentration versus time was linear. These linear relationships in Fig. 2 confirmed that the isomerization of linoleic acid is a zero-order reaction, where rate is independent of the concentration of the reactant. Kinetic modeling allows to derive basic information for the system in order to describe the reaction rate as a function of processing time, and thus predict changes in a particular oil during heat treatment.
Figure 3 displays the C18:2tt, C18:2tc and C18:2ct content as a function of processing time when heat treatment was performed at 272°C. The data were subjected to regression analysis using the zero-order reaction (Eq. 1 and 2).
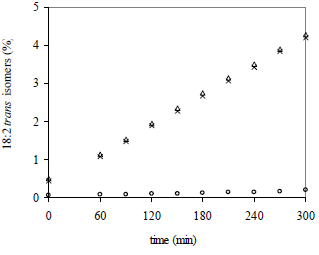
Figure 3. Trans isomers of linoleic acid as a function of processing time (deodorization temperature: 272°C). References: o C18:2tt, x C18:2tc, D C18:2ct.
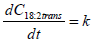 | (1) |
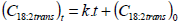 | (2) |
where Ct is the measured concentration value (g/ml), C0 is the initial concentration, t is the reaction time (min), and k is the reaction rate constant (g/ml.min).
The Arrhenius equation is usually applied to describe the reaction rate constant temperature dependence:
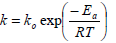 | (3) |
where ko is the pre-exponential factor (g/ml.min), Ea is the activation energy (J/mol) for the trans-18:2 isomer formation process, and R is the gas constant (8.314 J/mol.K).
Table 2 shows the values of the kinetics constants k obtained at different temperatures for the three C18:2 trans isomers. Values for 179 and 199°C are not reported because concentration measurements presented high dispersion, possibly due to errors in the chromatographic analyses as a consequence of the low concentration of trans isomers.
Table 2. Isomerization constants of linoleic acid 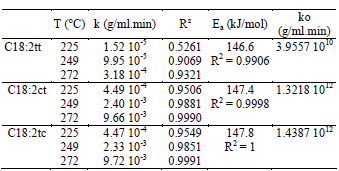
The values for the rate constant were higher for the formation of C18:2ct and C18:2tc than for C18:2tt. The k values for C18:2ct and C18:2tc were very similar, consistently with their concentration after the heat treatment (Table 1).
The goodness of fit of the experimental data to the model is measured with the correlation coefficient R2. The correlation coefficient values ranged from 0.90 to 1, except for C18:2tt content at 225°C which could be a consequence of its low concentration. Therefore, the formation of trans isomers can be correctly fitted with the zero-order reaction.
The activation energy for the trans isomer formation was calculated from the slope of the Arrhenius plot of the reaction rate constant of 225, 249 and 272°C, as shown in Figure 4. The Ea values were 146.6, 147.4 and 147.8 kJ/mol for C18:2tt, C18:2ct and C18:2tc respectively. The activation energies were comparable for the three isomers, but the pre-exponential factor of C18:2tt was lower than the corresponding factor for mono-trans C18:2 isomers. This explains why the content of mono-trans C18:2 isomers was higher than that of C18:2tt in all the sample measurements.
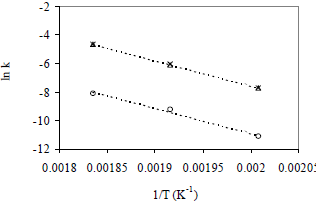
Figure 4. Arrhenius plot for the C18:2 trans isomer formation. References: o C18:2tt, x C18:2tc, D C18:2ct.
These results are compared to those reported by León Camacho et al. (2001) for the deodorization of sunflower oil using nitrogen as stripping gas instead of steam. The kinetic constants for the formation of the C18:2ct, C18:2tc and C18:2tt acids were 138.9, 152.72 and 116.7 kJ/mol, also using a zero-order reaction.
There are numerous references to the kinetics of trans isomer formation during deodorization, although the majority of these works reported first order reaction for linolenic isomerization. O'Keefe et al. (1993) analyzed soybean oil and reported that the loss of linolenic acid followed an apparent first-order kinetics and the activation energy was 82.1 kJ/mol. The formation of C18:3cct did not follow simple kinetics, and its apparent activation energy was 146.0 kJ/mol.
Wolff (1997) studied the formation of linolenic acid geometrical isomers in linseed oil when it was heated under vacuum at different temperatures (190-260°C) for several durations (2-16 h). At temperatures higher than 200°C, disappearance of linolenic acid followed a first-order kinetic.
Hénon et al. (1999) reported that the formation of trans linolenic and linoleic acid isomers followed a first-order reaction and the kinetic constant varied according to the Arrhenius law. They found isomerization constants for linoleic acid range from 2.5 10-4 to 150 10-4 h-1 when temperature varied between 210 and 270°C.
Gercar and Smidovnik (2002) performed a laboratory treatment of soybean oil under atmospheric pressure in the presence of air or nitrogen at different temperatures ranging from 160 to 250°C for 12 to 72 h. For both linoleic and linolenic acid, the reaction of formation of trans isomers followed a first-order reaction, and the rate constant of isomerization varied according to the Arrhenius law. The isomerization rate constant (at 250°C) was 7.39×10−3 h−1 for the linoleic acid (in absence of oxygen) and 0.87×10−1 h−1 for the linolenic acid.
The statistical results for the trans-C18:2 formation during the heat treatment of sunflower oil are summarized in Table 3. The analysis of variance (ANOVA) partitions the variability in the total trans-C18:2 content into separate components for each of the effects. In this case, three effects are statistically significant (with P-values less than 0.05): temperature, time and their interaction. Furthermore, R2 indicates that the fitted model explains 90.45% of the variability.
Table 3. Summary of the statistical analysis (ANOVA) for total C18:2 trans.
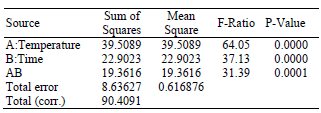
R-squared = 90.4473 %
Figure 5 shows a surface plot for the total trans-C18:2 formation during the heat treatment process using steam as stripping gas (0.01 kg/min) under atmospheric pressure. The results of the present work are in agreement with the published data reporting that residence time and operating temperature have the most important effect on the extent of trans isomers formation (Lambelet et al., 2003).
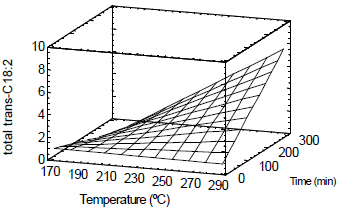
Figure 5. Surface plot. Total trans-C18:2 formation during deodorization process.
Kellens (1997) indicated that, in order to obtain deodorized oils with low levels of TFA (<1%), physical refining should be performed at 235-240°C. Taking into account the relative effect of temperature and time (Table 3), lower temperature and longer processing could be used in order to decrease TFA formation. Under the operation conditions reported in this work, it would be necessary to operate at temperatures lower than 250°C for a maximum of 2 h in order to accomplish an acceptable TFA value, considering that the sunflower oil contained an initial amount of trans isomers of ~1%.
IV. CONCLUSIONS
Heat treatment of sunflower oil, similar to deodorization were carried out by applying steam stripping at different temperatures ranging from 179 to 182°C for 5 h.
The results showed an increase in the relative percentage of trans linoleic acid with an increase in either time or temperature. The formation of trans linoleic acid isomers followed a zero-order reaction and the kinetic constants varied according to the Arrhenius' law. The activation energies for the formation of the acids C18:2ct, C18:2tc and C18:2tt were calculated as 147.4, 147.8 and 146.6 kJ/mol, respectively.
| NOMENCLATURE | |
| C | concentration (g/ml) |
| C18:1c | oleic acid |
| C18:1t | elaidic acid |
| C18:2 | linoleic acid |
| C18:3 | linolenic acid |
| Ea | activation energy (J/mol) |
| k | reaction rate constant (g/ml.min) |
| ko | pre-exponential factor |
| R | gas constant (8.314 J/mol K) |
| t | time (min) |
ACKNOWLEDGEMENTS
The authors thank the Universidad Nacional del Sur (UNS) and the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) for their financial support.
REFERENCES
1. Ackman, G.G., S.N. Hooper and D.L. Hooper, "Linolenic acid artifacts from deodorization of oils," J. Am. Oil Chem. Soc., 51, 42-49 (1974).
2. Aro, A., J. van Amelsvoort, W. Becker, M-A. van Erp-Baart, A. Kafatos, T. Leth and G. van Poppel, "Trans fatty acids in dietary fats and oils from 14 European countries: The TRANSFAIR Study," J. Food Compos. Anal., 11, 150- 160 (1998).
3. Ceriani, R. and A.J. Meirelles, "Formation of trans PUFA during deodorization of canola oil: A Study through computational simulation," Chem. Eng. and Proc., 46, 375-385 (2007).
4. Cook R., "Thermally induced isomerism by deodorization", INFORM, 13, 71-76 (2002).
5. FDA, FDA Acts to Provide Better Information to Consumers on Trans Fats, http://www.fda.gov/oc/ initiatives/transfat/ (2003).
6. Gercar, N. and A. Smidovnik, "Kinetics of geometrical isomerization of unsaturated FA in soybean oil," J. Am. Oil. Chem. Soc., 79, 495-500 (2002).
7. Han, S.N., L.S. Leka and A.H. Lichtenstein, "Effect of hydrogenated and saturated, relative to polyunsaturated, fat on immune and inflammatory responses of adults with moderate hypercholesterolemia," J Lipid Res., 43, 445-452 (2002).
8. Hénon, G., Z. Kemény, K. Recseg, F. Zwobada and K. Kovari, "Deodorization of Vegetable Oils. Part I: Modelling the Geometrical Isomerization of Polyunsaturated Fatty Acids," J. Am. Oil Chem. Soc., 76, 73-81 (1999).
9. IUPAC, Standard Methods for the Analysis of Oils, Fats and Derivatives, 7th. Ed., C. Paquot and Hautfenne (Ed.). International Union of Pure and Applied Chemistry, Blackwell Scientific Publications Inc, Oxford (1992).
10. Kellens M., Current Developments in Oil Refining Technology, Technical Report of Smet- Belgium, Antwerp (Belgium), 31-48 (1997).
11. Lambelet, P., A. Grandgirard, S. Gregoire, P. Juaneda, J.L. Sebedio and Bertoli C., "Formation of Modified Fatty Acids and Oxyphytosterols during Refining of Low Erucic Acid Rapeseed Oil," J. Agric. Food Chem., 51, 4284-4290 (2003).
12. León Camacho, M., M. Ruiz-Méndez and E. Graciani Constante, "Kinetics of the cis-trans isomerization of linolenic acid in the deodorization and/or physical refining of edible fats," Eur. J. Lipid Sci. Technol., 103, 85-92 (2001).
13. Medina-Juárez LA, N. Gámez-Meza, J. Ortega-García, J.A. Noriega Rodríguez and O. Angulo-Guerrero, "Trans fatty acid composition and tocopherol content in vegetable oils produced in Mexico," J. Amer. Oil Chem. Soc., 77, 721-724 (2000).
14. Mozaffarian D., E. Rimm, I. King, R. Lawler, G. McDonald and W. Levy, "Trans fatty acids and systemic inflammation in heart failure," Am. J. Clin. Nut., 80, 1521-1525 (2004).
15. Norris, F.A., Bailey's Industrial Oil and Fat Products, vol.3, 4th ed., Wiley-Interscience, New York (1985)
16. O'Keefe, S., V. Wiley and D. Wright, "Effect of temperature on linolenic acid loss and 18:3 Δ9 cis, Δ12 cis, Δ15 trans formation in soybean oil," J. Am. Oil Chem. Soc., 70, 915-917 (1993).
17. Wolf, R.L., "Heat-induced geometrical isomerization of a-linolenic acid: effect of temperature and heating time on the appearance of individual isomers," J. Am. Oil. Chem. Soc., 70, 425-430 (1997).
Received: May 6, 2009
Accepted: August 4, 2009
Recommended by Subject Editor: Ricardo Gómez.