Servicios Personalizados
Articulo
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. vol.40 no.3 Bahía Blanca jul. 2010
Improving emulsion separation: the collector material concept
C. J. Morales†,*, U. Riebel†, L. Zavarce‡ and N. M. Guzmán*
† Lehrstuhl Mechanische Verfahrenstechnik, Brandenburgische Technische Universität Cottbus, PF 101344, D-03013, Cottbus, Germany. moralesc@gmx.net, carlos.morales@ucv.ve, sekretrariat@mvt.tu-cottbus.de
‡ Quality Management Department Geohidra Consultores. Caracas, Venezuela litkalinazavarce@yahoo.es
* Chemical Engineering School. Universidad Central de Venezuela. P.O. Box 50656 - Sabana Grande, Caracas 1051-A, Venezuela nolides.guzman@ucv.ve
Abstract — A variety of materials have been used to investigate their applicability as so-called collector materials to improve the destabilization and separation of water in oil emulsions stabilized by a nonionic surfactant. The emulsion destabilization degree was determined by the extent of water and oil separated after centrifugation. The recovery of both phases was strongly dependent on the nature of the material, material/emulsion ratio, particle size, and contact time. By varying conditions, it is possible to increase the separation of water from 0% (without material) up to 95%.
Keywords — Collector Material. Emulsion Separation. Emulsion Destabilization. Coalescence. Sedimentation.
I. INTRODUCTION
Packings can improve the physical separation of immiscible phases according with its capacity to provide interfacial contact area and its preferential affinity for one of the phases. The wetting concept has been used in the last years to increase the separation in different processes involving emulsions.
Dezhi et al. (1999) studied demulsification of water-in-oil droplets with wetting coalescence materials using conventional stirred and packed columns. They tested four kinds of natural fibers and two kinds of inorganic materials, obtaining that natural fiber A, selected from shaves of wood showed the best performance in demulsification. The packed column showed much better performance in terms of demulsification efficiency and repeated use of the recovered oil phase for extracting cadmium in a simulated wastewater.
Ito et al. (2002) studied the rapid separation of n-decane oil droplets in the presence of sodium dodecyl sulfate (SDS) by a packed bed column with fibrous ferro-nickel slag (FS); the results were discussed in terms of electric interfacial interaction of both an n-decane droplet and a collector medium. They suggested that separation using surface characteristics is a promising method for the recovery of stable emulsified oil droplets.
Shin and Chase (2004) determined the wettability effect on the coalescence mechanism in fibrous filters. Experimental results from tests on coated glass rods were presented and flow of water-in-oil emulsion around the glass rods was observed. When the rod surface is wetting, the disperse phase coats the rod, whereas when the surface is non-wetting drops neither stick easily to the surface nor coat it, but slide around to the downstream side, where they are dragged off the rod.
Kapilashrami et al. (2004) investigated the substrates effect of the hydrophobic/hydrophilic character on the drying behaviour of dilute silicone oil-in-water emulsions by light microscopy and ellipsometry, and showed that emulsion droplets do not adsorb to the hydrophilic substrate. The hydrophobic substrate, on the other hand, destabilizes the emulsion and increases the coalescence rate. They suggested that destabilization is promoted by the strong interaction between the emulsion droplets and the hydrophobic substrate.
In this work, the effect of packing material on centrifugal separation of highly stable water-in-oil emulsions is studied. A fundamental characteristic of surfactants is their tendency to adsorb at interfaces in an oriented manner. Surfactant orientation and packing at the interface determines how the interface will be affected by the adsorption i.e. whether it will become more hydrophilic or more hydrophobic (Rosen, 2004).
The contact angle can not be quantified when the solid is formed by finely divided particles. In this case, if surfactant adsorption turns the solid more hydrophilic, it will disperse better in water after adsorption; but hydrophobic particles will either float or settle down of the water faster after surfactant adsorption.
Alternatively, the particles may be shaken with a mixture of equal volumes of water and a non-polar solvent (e.g., hexane). If the particles have become more hydrophobic by surfactant adsorption, they will disperse better in the non-polar phase than before; but if they have become more hydrophilic, they will disperse better in the aqueous phase (Rosen, 2004).
II. EXPERIMENTS AND RESULTS
A. Emulsion Formulation
Stable synthetic water-in-oil emulsions with 25, 50, 75 and 80% v/v water and 2% v/v surfactant were prepared. Paraffin oil and the surfactant highly soluble in oil, Sorbitan Monooleate (Span 80®) were previously mixed. Then, distillated water was added slowly (using a burette) during 5 min, while it was mixed with a Braun stirrer model MR-5550, at 13400 rpm. After adding all the water, the components were mixed for 5 more min. 500 mL of emulsion (water plus paraffin) was prepared in each run in a 600 mL beaker. Figure 1 shows the visual appearance of the 80% v/v water-in-oil emulsion.

Figure 1. Appearance of W/O emulsion stabilized by Span 80® (Vb = 500 mL, tm = 5 min)
B. Materials Pre Selection and Preliminary Test
Solids with different hydrophobicities were pre selected by a study of Zavarce (2006) and are described on Table 1. Hydrophobicities were estimated from the wettability observed when droplets of water and paraffin were placed on the material surface. Void fraction was calculated as the ratio between the free volume of 10 g of material (measured with water) and the measurable volume of the material in a graduated conic test tube of 15 mL volume.
Table 1. Characteristics of the Pre selected Materials 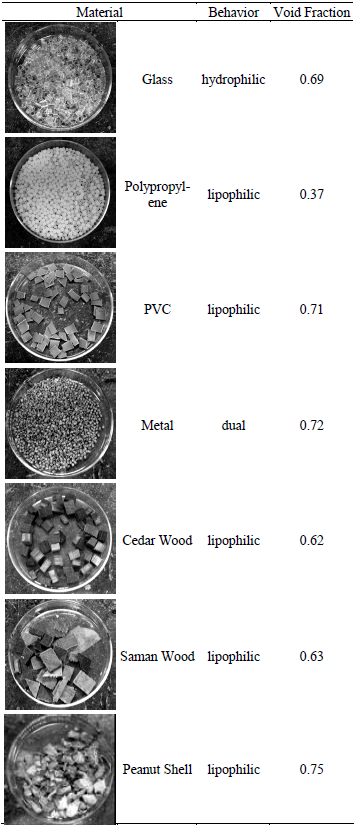
Figure 2 shows the peanut shell apparent wettability by water (left) and paraffin (right). It can be observed that the water droplet keeps its shape while the paraffin droplet spreads on the peanut shell surface, indicating that the material is preferentially wetted by paraffin; therefore, it can be defined as lipophilic. A hydrophilic material is defined as well wetted only by water, and dual material refers to the one that is wetted by both water and paraffin.

Figure 2. Water/Peanut Shell (left) and Paraffin/Peanut Shell (right) Wettability
Figure 3 shows an example of the phases distribution on a Sample. Samples with 9 g of emulsion (e) and 1 g of material (m) were mixed with a glass rod for some seconds in centrifuge test tubes and were centrifuged at 2600 rpm. Water-in-oil emulsion stability against both clarification and coalescence was measured as a function of centrifugation time.
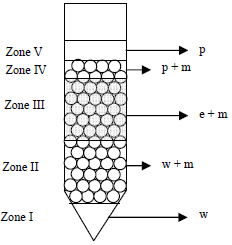
Figure 3. Example of the Phases Distribution on a Sample
Clarification is defined as the recovered paraffin volume (p) because the water droplets move to the bottom leaving clarified paraffin on the top. Coalescence is given as the recovered water volume (w). In the most general case, water is recovered in zones I and II (see Fig. 3), whereas paraffin is recovered in zones IV and V. For liquid phase volume determination in the zones II and IV it is necessary to consider the material void fraction.
The kinetics of water and paraffin separation in preliminary tests is shown in Fig. 4 and 5, respectively. Two statements are obtained from these results: (1) for some materials it is possible to increase the water and paraffin recovered percentage in comparison with the absence of material; (2) there is a big difference between Cedar wood and Saman wood curves for both water and paraffin recovery. Differences can also be observed in the case of polypropylene and PVC curves. Hence, the result depends not only on the nature of the material.
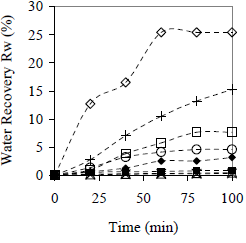
Figure 4. Effect of Centrifugation Time on Water Recovery in Preliminary Tests. Cm = 10% w/w. ( ) Cedar wood, (+) peanut shell, (
) Cedar wood, (+) peanut shell, ( ) polypropylene, (
) polypropylene, ( ) glass, (
) glass, ( ) Saman wood, (
) Saman wood, ( ) PVC, (×) metal, (
) PVC, (×) metal, ( ) without material
) without material
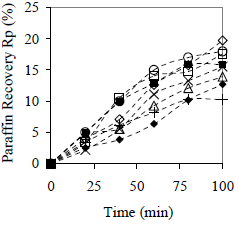
Figure 5. Effect of Centrifugation Time on Paraffin Recovery in Preliminary Tests. Cm = 10% w/w ( ) Cedar wood, (+) peanut shell, (
) Cedar wood, (+) peanut shell, ( ) polypropylene, (
) polypropylene, ( ) glass, (
) glass, ( ) Saman wood, (
) Saman wood, ( ) PVC, (×) metal, (
) PVC, (×) metal, ( ) without material
) without material
Comparing Fig. 4 and 5 it can be noticed that there is a stronger material influence on the coalescence process (Fig. 4) than on the clarification process (Fig. 5). Clarification (or sedimentation without coalescence) occurs even in absence of material, but a constant value of paraffin recovery is not reached in the time of the study.
Glass (highly hydrophilic) and peanut shell (highly lipophilic) were selected for the main tests, in order to understand the influence of type and size of material on the emulsion stability.
C. Effect of Material Type on Phases Recovery
Crushed glass and peanut shell were sieved and particles smaller than 0.89 mm were used. Samples with 9 g of emulsion and 1 g of material previously mixed with a glass rod were centrifuged at 2600 rpm for 2 h. Water and paraffin recovery were evaluated as a function of time. Each test was repeated three times and the average values and standard deviations were calculated to evaluate the repeatability.
The effect of using collector materials on water coalescence is reported in Fig. 6. As it is shown, both materials, the hydrophilic (glass) and the hydrophobic (peanut shell) improve the coalescence in comparison with the absence of collector materials; therefore, for this particle size and material ratio, peanut shell can be defined as a "good collector material" where water recovery reaches 86 ± 5%. In contrast, glass is a "poor collector material" with a water recovery of only 11 ± 2%.
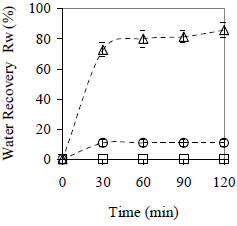
Figure 6. Effect of the Material Type on Average Water Recovery. Cm = 10% w/w, x < 0.89 mm. ( ) peanut shell, (
) peanut shell, ( ) glass, (
) glass, ( ) without material
) without material
In Fig. 6, glass and peanut shell particles were smaller than in the preliminary test, detecting faster coalescence for both materials. In this case, separation occurs above 30 min; but for larger particles (see Fig. 4) more time is required to reach the maximum recovery: 80 min for glass and more than 100 min for peanut shell. The particle size influences not only the coalescence rate but also the maximum amount of separated water, because for larger particles (see Fig. 4) the water recovery is hardly 15 % and 5% for peanut shell and glass, respectively. Besides the differences on wettability between both materials, the glass also settles faster than the peanut shell, which means a less effective contact between the material and the emulsion.
Results obtained for paraffin recovery are shown in Fig. 7. Only the lipophilic material promotes clarification by sedimentation itself, and 82% of paraffin is recovered in comparison with 54% for the hydrophilic material and without material. Clarification requires more time than coalescence; therefore, no maximum values are reached in 120 min. Observing clarification kinetics, two steps are present: a faster one, approximately during the first 30 min, that is controlled by the nature of the material (it is here where the material hydrophobicity is important). The second step is slower, and the rate of clarification is practically independent on the material nature.
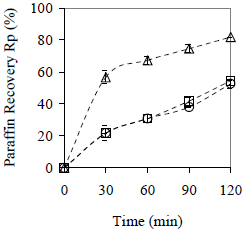
Figure 7. Effect of the Material Type on Average Paraffin Recovery. Cm = 10% w/w, x < 0.89 mm. ( ) peanut shell, (
) peanut shell, ( ) glass, (
) glass, ( ) without material
) without material
D. Effect of Collector Material Particle Size on Phases Recovery
To evaluate the effect of collector material particle size on separation observed in Fig. 4 and 6, peanut shell was ground and sifted through sieves of 0.89 and 2.38 mm. Four sizes were used including the half cuts (see Fig. 8).

Figure 8. Sizes of used Peanut Shell. (a) x < 0.89 mm, (b) 0.89 < x < 2.38 mm, (c) x > 2.38mm, (d) half peanut shell
Samples of emulsion with 10 % w/w of material were centrifuged up to 30 min, corresponding to the first kinetic step, at 2600 rpm. The volume of separated water was measured every 10 min and water recovery was calculated and reported in Fig. 9.
Figure 9. Effect of the Particle Size on Average Water Recovery. Material: peanut shell. ( ) x < 0.89 mm, (
) x < 0.89 mm, (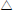 ) 0.89 mm < x < 2.38 mm, (
) 0.89 mm < x < 2.38 mm, ( ) x > 2.38 mm, (×) half shell, (
) x > 2.38 mm, (×) half shell, ( ) without peanut shell
) without peanut shell
Coalescence is strongly governed by material size. For sizes bigger than 0.89 mm the water recovery is lower than or equal to 8% whereas with particles smaller than 0.89 mm, the water recovery reaches 71%, which is over 8 times higher.
E. Emulsion Type and Collector Material Efficiency
W/O and O/W emulsions with 30% v/v water and 70% v/v paraffin were prepared using a surfactant mixture with 85% v/v of Sorbitan Monooleate (span 80®) and 15% v/v of Nonil Phenol Ethoxilated (N150®) to provide high stability. Only the mixing array was change to produce both emulsion types: for W/O emulsion, water was added to paraffin and for O/W emulsion, paraffin was added to water.
Two material particle sizes were selected: fine particles (x < 0.2 mm) and coarse particles (x > 0.8 mm). Samples with 1.5 g of peanut shell of each size were prepared in test tubes, 30 mL of W/O emulsion was added to each tube. An equivalent set was prepared with O/W emulsion. All tubes were centrifuged three times for 20 min, and after the third centrifugation they were monitored for 3 weeks. Tubes with pure emulsions were also centrifuged to demonstrate the emulsions stability. Fig. 10 shows the results after the third centrifugation. Between the second and the third centrifugation no changes were observed. Three weeks later, slight changes in sedimentation and coalescence were observed. The collector material effect is independent of the emulsion nature because there is an equivalent separation between tests with O/W emulsions (from (a) to (c) in Fig. 10) and the W/O emulsions (from (d) to (f) in Fig. 10). Once again, fine particles (tubes (a) and (d)) promote both sedimentation and coalescence better than the coarse particles (tubes (b) and (e)).
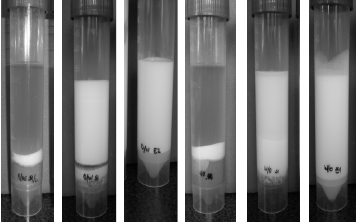
Figure 10. Effect of Emulsion Type on Collector Material Efficiency. (a) O/W emulsion and fine dry peanut shell, (b) O/W emulsion and coarse dry peanut shell, (c) pure O/W emulsion, (d) W/O emulsion and fine dry peanut shell, (e) W/O emulsion and coarse dry peanut shell, (f) pure W/O emulsion
F. Effect of Collector Material Contact Time on Phases Recovery
Water droplets were observed one day later on the walls of some test tubes. It was decided to evaluate the effect of the material contact time. Two cases were studied: in the first case, the samples were centrifuged for 20 min immediately after their preparation; in the second case, the samples were also centrifuged for 20 min but 24 h later. In both cases, centrifugation was set at 2600 rpm. Results are presented in Fig. 11.
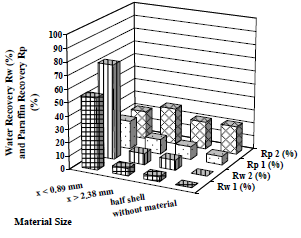
Figure 11. Effect of Material Contact Time on Average Water and Paraffin Recovery. Material: peanut shell. tc = 20 min.  Rw 1,
Rw 1,  Rw 2,
Rw 2,  Rp 1,
Rp 1,  Rp 21
Rp 21
Water recovery is strongly influenced by the collector material particle size; the smaller the particle size, the greater its effect on the coalescence process for both operational modes. Water recovery changes from 0 to 54% in the first operational mode, and from 0 to 72% in the second one.
The same behavior is observed on paraffin, but only in the first operational mode (paraffin recovery changes from 7 to 21%); nevertheless, when the centrifugation is made a day after the sample preparation, the paraffin recovery does not show an important variation (from 21 to 27%), and the amount of separated paraffin for all material sizes practically agrees with the experiments in which no material is used.
In order to find the maximum recovery, another test was carried out following the procedure previously described as "the second case", but repeating the cycle during several days. These results are shown in Fig. 12 and 13.
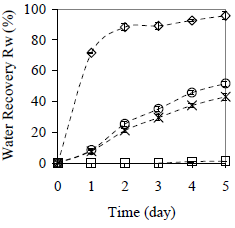
Figure 12. Effect of the Particle Size on Average Water Recovery with 20 min Centrifugation per Day. Material: peanut shell. ( ) x < 0.89 mm, (
) x < 0.89 mm, ( ) x > 2.38 mm, (×) half shell, (
) x > 2.38 mm, (×) half shell, ( ) without peanut shell
) without peanut shell
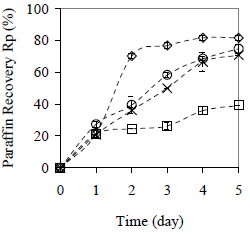
Figure 13. Effect of the Particle Size on Average Paraffin Recovery with 20 min Centrifugation per Day. Material: peanut shell. ( ) x < 0.89 mm, (
) x < 0.89 mm, ( ) x > 2.38 mm, (×) half shell, (
) x > 2.38 mm, (×) half shell, ( ) without peanut shell
) without peanut shell
The water and paraffin recovery is increased up to 96 and 82%, respectively, when smallest collector material size is used (x < 0.89 mm). Separation kinetics is similar as described in Fig. 7 and 8. For water, the rate of separation is increased when the size of the collector material diminish. For paraffin the same behavior occurs, but only after the first operational cycle (day 2), because for the first day, the influence of the material size in the paraffin recovery is not verified.
To evaluate separately the contribution of daily centrifugation and contact time, a sample with the finest particle size material was prepared and centrifuged for 20 min at 2600 rpm, only the first and last day. For comparison, Fig. 14 shows the case when daily cen trifugation was made. Contact time is responsible of emulsion destabilization, and centrifugation only helps the physical separation of the phases. At the last day both samples, with and without daily centrifugation, show the same separation of water and paraffin. Also, when centrifugation could help destabilizing the emulsion, it is clear that the contact time is the parameter that takes the control of the total effect.
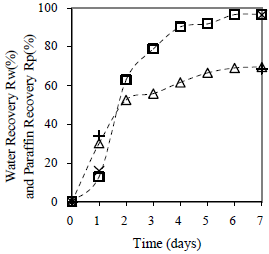
Figure 14. Effect of Contact Time on Average Water and Paraffin Recovery. Material: peanut shell, Cm = 5% w/w, tc = 20 min/day, x < 0.89 mm.: ( ) Rw 1, (+) Rw 2, (
) Rw 1, (+) Rw 2, ( ) Rp 1, (×) Rp 22
) Rp 1, (×) Rp 22
G. Emulsion Water Content and Collector Material Effectiveness
W/O emulsions with fw = 0.25, 0.50 and 0.75 were prepared and stabilized with span 80®. The emulsifier content was the same for the three formulated emulsions. Suspensions with 90% w/w of W/O emulsion and 10% w/w of collector material (peanut shell) in size smaller than 0.89 mm were centrifuged at rpm 2600 until the recovered amount of both phases was constant. The tests were repeated three times, and the standard deviation was calculated and reported. Results are shown in Fig. 15 and 16.
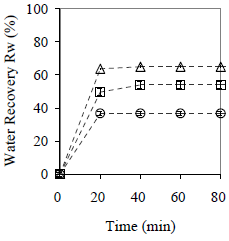
Figure 15. Effect of Emulsions Water Content on Effectiveness of the Collector Material to promote Coalescence. Material: peanut shell, Cm = 10% w/w, x < 0.89 mm. ( ) fw = 0.75, (
) fw = 0.75, ( ) fw = 0.50, (
) fw = 0.50, ( ) fw = 0.25
) fw = 0.25
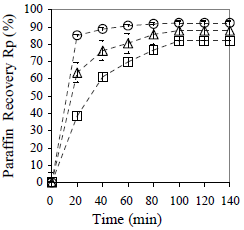
Figure 16. Effect of Emulsions Water Content on Effectiveness of the Collector Material to Promote Sedimentation. Material: peanut shell, Cm = 10% w/w, x < 0.89 mm. ( ) fw = 0.75, (
) fw = 0.75, ( ) fw = 0.50, (
) fw = 0.50, ( ) fw = 0.25
) fw = 0.25
An emulsion with low disperse phase content tends to be, by nature, more stable than an emulsion with high disperse phase content. In these experiments, the highest water recovery (Rw = 65%) is reached for fw = 0.50, whereas the smallest value was obtained for fw = 0.25 (Rw = 37%).
The results are attributed to differences in the HLB required because all the emulsions were formulated at HLB constant. It seems to be that, for a given particle size, there is an amount of peanut shell that optimizes the water recovery, and that depends on the emulsion water content. In terms of separation rate, the process becomes slower when the water content of the emulsion increases, but it is in general faster than the clarification process. The paraffin recovery diminishes when the emulsion water content increases, and values of 92, 88 and 82% v/v are obtained for fw = 0.25, 0.50 and 0.75, respectively.
Separation as a function of emulsion water content and collector material amount is compared in Fig. 17 and 18. Tests at 0, 5 and 10% w/w of collector material were made. The samples were centrifuged per consecutive 20 min periods, and the separation of water and paraffin was registered until the last 3 values were constant for each case.
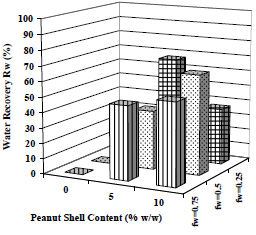
Figure 17. Effect of Collector Material Content on Coalescence. Material: peanut shell, Cm = 0, 5 and 10% w/w, x < 0.89 mm.  fw = 0.75,
fw = 0.75,  fw = 0.50,
fw = 0.50,  fw = 0.25
fw = 0.25
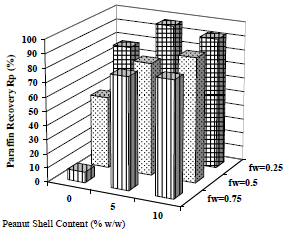
Figure 18. Effect of Collector Material Content on Sedimentation. Material: peanut shell, Cm = 0, 5 and 10% w/w, x < 0.89 mm.  fw = 0.75,
fw = 0.75,  fw = 0.50,
fw = 0.50,  fw = 0.25
fw = 0.25
Figure 17 shows that there is no water separation in the absence of collector material, and when the emulsion concentration increases, more collector material is required: when 5% w/w of material is used, water recovery is 67% v/v for the emulsion with 25% of water, but to obtain a similar recovery (65% v/v) in a more concentrated emulsion (with 50% of water), 10% w/w of material is required. In clarification case (corresponding to Fig. 18), the same trend is observed, but clarification occurs spontaneously in all cases, including also those without collector material.
H. Proposed Mechanism
It seems that the collector material does not only help on the physical separation of the phases, but also promotes emulsion destabilization: the surfactant finds a new state of balance and leaves the water-oil surface, allowing coalescing of water droplets. The new state also must satisfy the surfactant double affinity which is preferentially soluble in oil.
Theory of the surfactant-water-oil (SWO) systems behavior leads us to formulate a hypothesis: the surfactant leaves the water-oil surface and is adsorbed on the solid surface by the tail side because its affinity to lipophilic surfaces. When the emulsion is broken, water can coalescence and settle. The surfactant leaves the head in contact with the free water that is within the collector material at the bottom of the test tubes, satisfying its amphiphilic character. Thus, destabilization would occur if there is enough interface area to allow the adsorption of all the surfactant.
To support the proposed mechanism, complementary test were made with particles smaller than 0.71 mm. Three samples with 1.5 g of peanut shell were prepared in test tubes; and two samples of these were submerged for 10 days in water and water plus 8% v/v surfactant Span 80® (previously mixed), respectively. After that, the excess of liquid was removed with absorbent paper from the so-called "wetted peanut shell". The material that was not submerged in liquid is called "dry peanut shell". The water as well as the surfactant could be adsorbed in the peanut shell; in which case, the capacity of the peanut shell to break the emulsion would have to fall when it is compared with the result reached with dry shell.
W/O emulsion with 80% v/v water and 20% v/v paraffin was prepared using Sorbitan Monooleate (span 80®) to provide high stability. 40 mL of W/O emulsion was added to test tubes within peanut shell (wetted with water, wetted with water/surfactant, and dry). After 10 days, all tubes were centrifuged at 2600 rpm for 5 min.
Dry particles promote a significant better separation than those that previously were submerged in water/surfactant mixture (total separation of 93% vs. 15%), indicating a partial exhaustion of the peanut shell in the second case; but when water wetted peanut shell is used, there is no significant differences in separation (91%) in comparison with the dry peanut shell sample result. The hypothesis of partial saturation of the solid via surfactant adsorption is supported and although the water could also be adsorbed, it is clear that the surfactant adsorption occurs and is the one which promotes emulsion destabilization.
Surfactant adsorption occurs only in presence of water, because when peanut shell previously wetted with pure Span 80® was tested in a similar experiment, water recovery did not diminish in comparison with the case when dry peanut shell was used. In presence of water, surfactant can satisfy its double affinity by adsorption on the solid surface that is lipophilic.
III. CONCLUSIONS
- In absence of collector material, the clarification process remarkably depends on emulsion water content, and concentrated emulsions are much more stable against clarification. When the collector material is used, clarification is increased for all water content ranges and paraffin recovery is independent of water content.
- Hydrophilic and hydrophobic materials can improve the coalescence of water in W/O emulsions, but when hydrophobic materials are used, the effect is more significant.
- Some collector materials as peanut shell not only help on the physical separation of the phases but also promote coalescence.
- Effect of collector materials on coalescence depends on the particle size. Both, rate of coalescence and amount of separated water increase when the collector material particle size is reduced.
- The optimal amount of collector material for improving emulsion destabilization depends on the emulsion water content. Concentrated emulsions required more collector material than the diluted ones.
- The effect of collector materials is independent of the emulsion nature type and can destabilize both W/O and O/W emulsions.
- A suggested mechanism that could explain the collector material effect, is considering that coalescence occurs because the surfactant finds a new state of balance in which it leaves the water-oil surface and is adsorbed on the solid surface by the tail side, leaving the head in contact with the formed free water and satisfying its double affinity.
- It is recommended to evaluate other materials that have lipophilic character and porosity to search for better performing materials, such as coal or activated carbon.
| NOMENCLATURE | |
| Cm | material content (%w/w) |
| Cs | surfactant concentration (%v/v) |
| fw | water fraction (-) |
| m | material |
| p | paraffin |
| Rp | paraffin recovery (%v/v) |
| Rw | water recovery (%v/v) |
| T | temperature (°C) |
| tc | centrifugation time (min) |
| tm | mixing time (min) |
| vc | centrifugation velocity (rpm) |
| vm | mixing velocity (rpm) |
| w | water |
| x | Particle size (microns) |
1 Rw 1 and Rp 1: recovery when centrifugation is carried out immediately after sample preparation; Rw 2 and Rp 2: recovery when centrifugation is carried out 24 h after sample preparation.
2 Rw 1 and Rp 1: daily centrifugation, Rw 2 and Rp 2: centrifugation at the first and last day
ACKNOWLEDGEMENTS
The authors wish to thank CDCH (Humanistic and Scientific Development Center): Projects PI-08-00-6129-2005 and PI-08-00-6127-2005, and DAAD (German Academic Exchange Service) for the economic support of this research.
REFERENCES
1. Dezhi, S., J. Chung, D. Xiaodong and Z. Ding, "Demulsification of Water in Oil Emulsion by Wetting Coalescence Materials in Stirred and Packed Columns," Colloids and Surfaces A: Physicochemical and Engineering Aspects, 150, 69-75 (1999).
2. Ito, H., H. Hayashi and H. Sasaki, "Rapid Separation of Oil Particles from Low-Concentrated O/W Emulsions in the Presence of Anionic Surfactants Using Fibrous Slag," Journal of Colloid and Interface Science, 252, 214-221 (2002).
3. Kapilashrami, A., K. Eskilsson, L. Bergström and M. Malmsten, "Drying of Oil in Water Emulsions on Hydrophobic and Hydrophilic Substrates," Colloids and Surfaces A: Physicochemical and Engineering Aspects, 233, 155-161 (2004).
4. Rosen, M. Surfactants and Interfacial Phenomena. John Wiley & Sons, Inc., New Jersey (2004)
5. Shin, C. and G. Chase, "The Effect of Wettability on Drop Attachment to Glass Rods," Journal of Colloid and Interface Science, 272, 186-190 (2004).
6. Zavarce, L. Utilización del Concepto de Materiales Colectores para Mejorar la Cinética de la Separación del Agua en Emulsiones de Agua en Aceite, Chemical Engineering Thesis, Universidad Central de Venezuela (2006).
Received: May 11, 2009
Accepted: July 24, 2009
Recommended by Subject Editor: Ricardo Gómez












