Servicios Personalizados
Articulo
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.32 n.2 Bahía Blanca abr./jun. 2002
Hydroformylation of oct-1-ene by supported aqueous phase catalysis: Influence of catalytic complex and preparation method of the supports
U. J. Jáuregui-Haza*1, A. M. Wilhelm2, H. Delmas2
1Centro de Química Farmacéutica, Apdo. 16042, C. Habana, Cuba. E-mail: ulises@cqf.co.cu
2Ecole Nationale Supérieure d'Ingénieurs de Génie Chimique, 18 chemin de la Loge, 31078 Toulouse, France
*Author to whom correspondence should be addressed
Abstract — Four silica samples were evaluated as supports in the hydroformylation of oct-1-ene by Supported Aqueous Phase Catalysis (SAPC) using [Rh2(µ-StBu)2(CO)2(TPPTS)2] and [Rh(COD)Cl]2/TPPTS as catalysts. Adsorption studies of the [Rh(COD)Cl]2/TPPTS complex from aqueous solutions on the supports were carried out at 25 °C. The isotherms obtained were correlated by several models, among which the Fowler-Guggenheim/Jovanovic-Freundlich equation was found to be the most satisfactory. The influence of the nature of catalytic complex and of the methods for supporting the catalyst on the silica conversion was also studied.
Keywords — Supported Aqueous-Phase Catalysis (SAPC), hydroformylation, oct-1-ene, silica, rhodium
1. Introduction
The recent development of Supported Aqueous Phase Catalysis (SAPC) opened the way to hydroformylate very hydrophobic alkenes such as octene, dicyclopentadiene or oleyl alcohol with water-soluble catalysts (Arhancet et al., 1989). But SAPC is still used with empirical rules, because the mechanisms governing the interactions between complex, water and solid surface are not well elucidated. For this reason, it is necessary to study in depth the adsorption of water soluble ligands and catalytic complexes onto inorganic supports, as an important step in the preparation of catalyst-support complex and the understanding of these catalytic properties. Recently, the adsorption of aqueous solutions of benzenesulfonic acid, 3, 3', 3''-phosphinidynetris-, trisodium salt (TPPTS), the most used hydrosoluble ligand in biphasic catalysis, and di (µ-tertiobutylthiolato) dicarbonyl, bis (benzenesulfonic acid, 3, 3', 3''- phosphinidyne-tris-, trisodium salt) dirhodium ([Rh(µ- tBu)(CO)(TPPTS)]2 or C1, M.W.= 1577.04) on different silica supports was reported (Jáuregui-Haza et al., 2001a). Although C1 and di (1,5 cyclo-octadiene) dichloro dirhodium, benzenesulfonic acid, 3, 3', 3''- phosphinidyne-tris-, trisodium salt ([Rh(COD)Cl]2/TPPTS2 or C2, M.W. = 1629.93) had been used for hydroformylating oct-1-ene by biphasic catalysis or SAPC (Chaudhari et al., 1995; Deshpande et al., 1996; Kalck et al., 1998; Jáuregui-Haza et al., 2001b), it was necessary to obtain the adsorption isotherms for this second complex and to compare the performances of these two supported catalysts in the same operating conditions.
On the other hand, several methods have been used for preparing the SAP catalysts. According to the preparation procedure, the methods can be classified into two groups : i) indirect method, when the support is first impregnated with the hydrosoluble catalytic complex, then dried and rehydrated before use (Arhancet et al., 1989, 1990, 1991a; Choplin et al., 1998; Guo et al., 1991; Horvath, 1990) ; ii) direct method : the support, catalytic complex and water are mixed at the same time in the reaction system (Arhancet et al., 1991b; Kalck et al., 1998). In general, the best conversions of hydroformylation of alkenes by SAPC have been obtained when the SAP catalyst was prepared by indirect method.
This paper deals with the hydroformylation of oct-1- ene by SAPC using C1 and C2 and four silica samples as supports. Adsorption studies of the C2 from aqueous solutions on the supports were carried out at 25 °C; the influence of the method of preparation of the supported catalyst was also investigated. Finally, the catalytic performances of this catalyst were evaluated and compared to those of C1.
2. Experimental section
TPPTS was supplied by Rhône-Poulenc. The complex C1 was synthesized as reported before (Kalck et al., 1988). The other chemicals and solvents were purchased from Aldrich and SDS, and used without further purification. All the reactions and adsorption experiments were carried out under an inert atmosphere (Argon or Nitrogen).
Four supports were used to prepare SAP catalysts: the Sipernat silica samples DS22 and DS50 (from Degussa) and two SDS silica samples (S60 and S200). Their BET surface area, pore volume, and average pore sizes were measured by nitrogen sorptometry, using a NOVA-1000 version 3.70 analyser. Particle size measurements were obtained on a Mastersizer MALVERN S. Table 1 shows the physical properties of the adsorbents.
The adsorption essays were carried out as described elsewhere (Jáuregui-Haza et al., 2001a). A fixed amount of silica (0.5 g) and 6 mL of an aqueous solution of C2 were placed in a 50 mL glass-stoppered flask and shaken at 100 rpm for 24 hours in a thermostated oscillating bath (JULABO-SW-20C). The aqueous solutions of C2 were prepared in deaerated water. The initial concentrations of solutions were in the range of 8.4 10-5 -0.549 g/g of solution, which includes the concentration ranges reported in the works on SAPC and biphasic catalysis (Arhancet et al., 1989, 1990, 1991; Chaudhari et al., 1995; Deshpande et al., 1996; Dos Santos et al., 1998; Guo et al., 1991; Herrmann and Kohlpainter, 1993; Horvath, 1990; Kuntz, 1981; Monteil, 1993). Preliminary experiments had shown these adsorption processes reached equilibrium within 12 hrs. Each experiment was duplicated under identical conditions. The concentration of Rh in the aqueous phase was determined by atomic absorption spectrometry (328.1 or 343.5 nm) with a VARIAN AA275 spectrometer.
The SAP catalysts were prepared by direct and indirect methods. In the second case, the impregnation step involved adsorption of an aqueous solution of C1 or C2 as described above, followed by the drying of the support under reduced pressure at 40°C during 24 hrs.
Catalytic tests were carried out in a 150 mL stainless steel stirred autoclave heated by a thermostatic oil bath. In a typical run, 2.26x10-5 mol of C1 or C2, an excess of TPPTS (P/Rh = 6, Jáuregui-Haza et al., 2001b), 2.3 g of the support, 57 mL of toluene, 2.55x10-2 mol of oct-1-ene and the amount of permuted water necessary to reach the desired hydration percentage (Jáuregui-Haza et al., 2001b) were used. The reactor was pressurized with 5 bar of an equivalent mixture of hydrogen and carbon monoxide and heated to 80 °C. After 3 hrs, unless otherwise stated, the gas mixture feed was stopped and the reactor was cooled down to room temperature. Then, the reactor was depressurized and the liquid and solid phases were separated by filtration. The organic phase was analyzed by gas phase chromatography on a Carlo Erba HRGC 5160 chromatograph equipped with a flame ionization detector and a capillary column Alltech Econopac FFAP (30 m; 0.53 mm; 1.2 µm), Tdet = 200 °C, PH2 = 0.45 bar.
3. Results and discussion
3.1 Adsorption isotherms
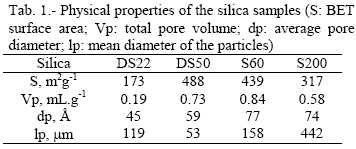
Experimental results of equilibrium adsorption isotherms of C2 are plotted in Fig. 1 (experimental data available by request). The average relative error on the measured concentration in the liquid phase was 4.05 % (with a higher value of 6.97 %). In the same figure, for comparison, the adsorption isotherms obtained previously (Jáuregui-Haza et al., 2001a) for C1 are also shown. In general, high capacities are obtained for both complexes on all supports. The adsorption capacity is higher for C1 than for C2 and for the both complexes it increases with an increase in total pore volume of the support (DS22 < S200 < DS50 < S60). The differences in adsorption capacity might be explained by the differences in molecular size of C1 and C2 as well as by the possible different interactions between the ligands and support surface.
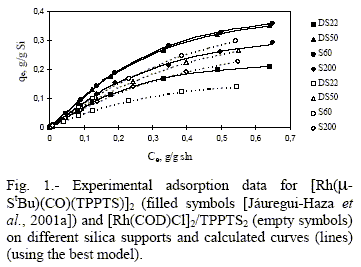
Different isotherm models have been used to correlate isotherm data for C2: the Langmuir model (Langmuir, 1916), for homogeneous surfaces without lateral interactions; the model (Fowler and Guggenheim, 1965), for homogeneous surfaces with lateral interactions; the Jovanovic-Freundlich model (Quiñones and Guiochon, 1996), for heterogeneous surfaces without lateral interactions and the Fowler-Guggenheim/Jovanovic- Freundlich model (Quiñones and Guiochon, 1998), for heterogeneous surfaces with lateral interactions. The equations of these four isotherm models are displayed in Tab. 2.
Fitting of the adsorption isotherm models to the experimental data was performed using a corrected Newton algorithm. The procedure calculates the values of the isotherm parameters which minimize the average standard error of estimation (SEE):
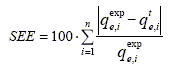 | (1) |
where ![]() are the elements of the vector
are the elements of the vector ![]() containing the given experimental adsorbed phase concentrations at equilibrium,
containing the given experimental adsorbed phase concentrations at equilibrium, ![]() are the corresponding theoretical values calculated by the model and n is the number of data points.
are the corresponding theoretical values calculated by the model and n is the number of data points.
The selection of the most adequate model was performed using Fisher's test. The model selected exhibited the highest value of the Fisher parameter Fcalc (Ajnazarova and Kafarov, 1985):
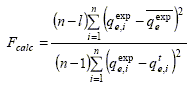 | (2) |
where ![]() is the mean value of the vector
is the mean value of the vector ![]() and l is the number of adjusted parameters of the model.
and l is the number of adjusted parameters of the model.
Table 3 summarizes the results of the nonlinear regression analysis. In general, the agreement of the models with the data is good as SEE and Fcalc values show. The Fowler-Guggenheim/Jovanovic-Freundlich model was found to best fit C2 data, while Fowler's model was found to be the most satisfactory for C1 (Jáuregui-Haza et al., 2001a). Figure 1 shows the comparison between experimental adsorption data and calculated values.
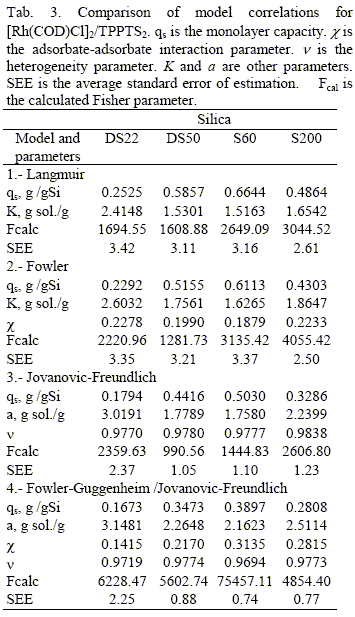
The increase of adsorption capacity whith icreasing porosity, could be explained by the possible adsorbateadsorbate interaction (Jaroniec and Madey, 1998). In this sense, both Fowler and Fowler-Guggenheim/Jovanovic- Freundlich equations, behave rather similarly (Tab. 3). The obtained values of χ show the importance of adsorbate-adsorbate interactions in the sorption process. It is well known, that positive values of χ imply that attraction forces between molecules predominate in the adsorbed layer (Fowler and Guggenheim, 1965). Regarding the surface heterogeneity, we can observe that the parameter ν in Jovanovic-Freundlich and Fowler- Guggenheim/Jovanovic-Freundlich models is close to unity for all supports. When the heterogeneity parameter is equal to unity, as for the systems studied, adsorption takes place on homogeneous surface.
3.2 Catalytic tests
From the best obtained model of adsorption isotherm, the needed initial concentration of the Rh-complex in aqueous solution was calculated for preparing SAP catalyst by the indirect method as described in the experimental section. Figure 2 shows the results of catalytic tests for both Rh-complexes when the indirect method of preparation of the SAP catalyst was used. The experimental average relative errors for the conversion and linearity were 5.24 % and 4.12 %, respectively. Regarding the conversion, C1 is more performance than C2. However, the linearity of the product, expressed as the percentage of lineal aldehyde out of the total amount of obtained isomers, was not dependent of the nature of Rh-complex. The influence of physical properties of the supports on the performance of oct-1-ene hydroformylation was discussed elsewhere (Jáuregui- Haza et al., 2001b). According to the size of pores SAPC could take place either at the surface of the pores, as described by Arhancet et al. (1990) and Horvath (1990), or at the external surface of the particle with a storage of TPPTS and catalytic complex in the filled pores. When the pores are full filled the SAPC can operate efficiently onto the external surface, stabilising the conversion in a relatively wide range of support hydration (Jáuregui- Haza et al., 2001b).
Fig. 2 .- Influence of the nature of Rh-complex on conversion and linearity for oct-1-ene hydroformylation after 3 hrs
reaction (white: [Rh(COD)Cl]2/TPPTS2; black: [Rh(µ-StBu)(CO)(TPPTS)]2; *significant difference for p < 0.05).
Figure 3 displays the influence of the preparation method of SAP catalyst on the conversion and linearity with C1. The indirect method resulted better than direct method only for the support S60, being the conversion statistically different for both methods. Considering that direct method is of easier implementation than the indirect one, the use of the first procedure is recommended with C1 for the hydroformylation of oct- 1-ene with the studied silica supports.
Fig. 3 .- Influence of the preparation method of SAP catalysts on conversion and linearity with [Rh(µ-StBu)(CO)(TPPTS)]2
after 3 hrs reaction (white: direct method, black: indirect method, * significant difference for p < 0.05)
In the case of C2, the hydroformylation did not take place after three hours when SAP catalyst was prepared by the direct method. A complementary experiment, carried out with DS22 support for 18 h, yielded a 4.6 % conversion with this complex, whereas with the C1, the conversion was 87.9 %. This behavior can be explained by the kinetics of formation of C2 from solid [Rh(COD)Cl]2 and TPPTS. Total dissolution of [Rh(COD)Cl]2 in the presence of TPPTS occurs after 30 min. When the direct method is used for the preparation of SAP catalyst, water adsorption onto silica might take place faster than complex formation, affecting the course of the reaction. Therefore, this method is not recommended for C2.
4. Conclusions
Adsorption studies of the [Rh(COD)Cl]2/TPPTS complex from aqueous solutions on the four silica supports at 25 °C showed a high capacity of adsorption on all supports, related to the total pore volume of the support. The isotherms were correlated by several models, among which the Fowler- Guggenheim/Jovanovic-Freundlich equation was found to be the most satisfactory.
On the other hand, the catalytic activity of the SAP catalyst prepared by the direct or the indirect method was higher with [Rh(µ-StBu)(CO)(TPPTS)]2 complex than with [Rh(COD)Cl]2/TPPTS.
Finally, the direct method for preparing SAP catalyst with [Rh(COD)Cl]2/TPPTS is not recommended due to the apparent slow kinetics of [Rh(COD)Cl]2 dissolution in water whereas it gives good results (as good as the indirect method) with [Rh(µ-StBu)(CO)(TPPTS)]2 complex .
Acknowledgements
The authors thank Prof. Philippe Kalck and Dr. Michel Dessoudeix for helping in the synthesis and characterization of [Rh(µ-StBu)(CO)(TPPTS)]2 ; Dr. Béatrice Biscans and Prof. Fernand Rodriguez for helping in characterization of silica ; DEGUSSA for the samples of silica and Rhône-Poulenc for the TPPTS. U.J.J.H. expresses his gratitude to ALFA-Programme of the European Community and INP-ENSIGC for the financial support of his travel and stay at the ENSIGC.
References
1. Ajnazarova, S. L.; Kafarov, V. V. Metodi Optimisatsi Eksperimenta v Khimicheskoy Teknologui; Vishaia Shkola: Moscow (1985).
2. Arhancet, J. P.; Davis, M. E.; Hanson, B. E. "Supported Aqueous-Phase Rhodium Hydroformylation Catalysts. 1. New Methods of Preparation". J. Catal., 129, 94-99 (1991a).
3. Arhancet, J. P.; Davis, M. E.; Hanson, B. E. "Supported Aqueous-Phase Rhodium Hydroformylation Catalysts. 2. Hydroformylation of linear, terminal and internal olefins". J. Catal., 129, 100-105 (1991b).
4. Arhancet, J. P.; Davis, M. E.; Merola, S. S.; Hanson, B. E. "Hydroformylation by supported aqueous-phase catalysis: a new class of the heterogeneous catalysts". Nature, 399, 454-455 (1989).
5. Arhancet, J. P.; Davis, M. E.; Merola, S. S.; Hanson, B. E. "Supported Aqueous-Phase Catalysts". J. Catal. 121, 327-339 (1990).
6. Chaudhari, R. V.; Bhanage, B. M.; Deshpande, R. M.; Delmas, H. "Enhancement of interfacial catalysis in a biphasic system using catalyst-binding ligands". Nature, 373, 501-503 (1995).
7. Choplin, A.; Dos Santos, S.; Quignard, F.; Sigismondi, S.; Sinou, D. "Palladium(0) allylic alkylation in a two-phase system or with a supported aqueous phase catalyst". Catal. Today, 42, 471-478 (1998).
8. Deshpande, R. M.; Purwanto; Delmas, H.; Chaudhari, R. V. "Kinetics of hydroformylation of 1-octene using [Rh(COD)Cl]2-TPPTS complex catalyst in a two-phase system in the presence of a cosolvent". Ind. Eng. Chem.Res., 35, 3927-3933 (1996).
9. Dos Santos, S.; Tong, Y.; Quignard, F.; Choplin, A.; Sinou, D.; Dutasta, J. P. "Supported Aqueous-Phase Palladium Catalysts for the Reaction of Allylic Substitution: Towards and Understanding of the Catalytic System". Organometallics, 17, 78-89 (1998).
10. Fowler, R.; Guggenheim, E. A., Statistical Thermodynamics, Cambridge Univ. Press: Cambridge (1965).
11. Guo, I.; Hanson, B. E.; Tóth, I.; Davis, M. E. "Hydroformylation of 1-hexene with Pt(m- C6H4SO3Na)3)2Cl2 and its tin chloride analogue on a controlled-pore glass". J. Mol. Catal., 70, 363- 368 (1991).
12. Herrmann, W. A; Kohlpainter, C. W. "Water-Soluble Ligands, Metal Complexes, and Catalysts: Synergism of Homogeneous and Heterogeneous Catalysis". Angew. Chem. Int. Ed. Engl., 32, 1524- 1544 (1993).
13. Horvath, I. T. "Hydroformylation of Olefins with the Water Soluble HRh(CO)[P(m-C6H4SO3Na)3]3 in Supported Aqueous-Phase. Is it Really Aqueous ?" Catal. Lett., 6, 43-48 (1990).
14. Jaroniec, M.; Madey, R. Physical Adsorption on Heterogeneous Solids; Elsevier: Amsterdam, 1998.
15. Jáuregui-Haza, U. J.; Wilhelm, A. M.; Delmas; Canselier, J. P. "Adsorption of benzenesulfonic acid, 3, 3', 3''-phosphinidynetris-, trisodium salt and di (µ-tertiobutylthiolato) dicarbonyl, bis (benzenesulfonic acid, 3, 3', 3''-phosphinidynetris-, trisodium salt) dirhodium from aqueous solutions on silica". J. Chem. Eng. Data, 46, 281- 285 (2001a).
16. Jáuregui-Haza, U. J.; Dessoudeix, M.; Kalck, Ph.; Wilhelm, A. M.; Delmas, H. "Multifactorial analysis in the study of hydroformylation of oct-1- ene using supported aqueous phase catalysis". Catalysis Today, 66, 297-302 (2001b).
17. Kalck, Ph.; Escaffre, P.; Serein-Spirau, F.; Thorez, A.; Besson, B.; Coleuille, Y.; Perron, R. "Watersoluble dinuclear rhodium complexes as catalytic precursors for the hydroformylation of alkenes in a two phases system". New J. Chem., 12, 687-690 (1988).
18. Kalck, Ph.; Miquel, L.; Dessoudeix, M. "Various approaches to transfers improvement during biphasic catalytic hydroformylation of heavy alkenes".Catalysis Today, 42, 431-440 (1998).
19. Kuntz,E. U. S. Patent 4,248,802 to Rhône-Poulenc Chimie de Base (1981).
20. Langmuir, I. "Constitution and Fundamental Properties of Solids and Liquids. I. Solids". J. Am. Chem. Soc., 38, 2221-2295 (1916).
21. Monteil, F. Carbonylation de l'Oct-1-ène et du Bromobenzene Catalysée par des Complexes Hydrosolubles du Palladium ou du Rhodium; Thèse de Doctorat de l'INP: Toulouse (1993).
22. Quiñones, I.; Guiochon, G. "Derivation and Application of a Jovanovic-Freundlich Isotherm Model for Single-Component Adsorption on Heterogeneous Surfaces". J. Colloid Interface Sci., 183, 57-67 (1996).
23. Quiñones, I.; Guiochon, G. "Extension of a Jovanovic- Freundlich Isotherm Model to Multicomponent Component Adsorption on Heterogeneous Surfaces". J. Chromatogr. A., 796, 15-40 (1998).
Received: April 5, 2001.
Accepted for publication: May 22, 2001.
Recommended by Subject Editor G. Meira.












