Servicios Personalizados
Articulo
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.32 n.2 Bahía Blanca abr./jun. 2002
Gas-phase β-pinene isomerization over acid-activated bentonite
E.L. Foletto†, A. Valentini ‡, L.F.D. Probst‡, and L.M. Porto†
† Departamento de Engenharia Química e Engenharia de Alimentos
‡ Departamento de Química
Universidade Federal de Santa Catarina, C. P. 476 - 88040-900, Florianópolis/SC, Brasil
efoletto@hotmail.com
Abstract —The kinetics of the gas-phase isomerization of β-pinene over an acid-activated bentonite clay has been investigated in a fixed-bed continuous flow reactor, at atmospheric pressure between 58 and 90 oC. The major isomers produced were camphene, α-pinene and limonene. The reaction rates of β-pinene conversion and the apparent activation energy were determined. The kinetics of β-pinene isomerization was described by zero-order kinetics with an activation energy of 30-35 kJ/mol for the major products formed.
Keywords — β-pinene, isomerization, acidactivated bentonite.
I. INTRODUCTION
Monoterpenes are widely distributed in nature, and occur in nearly all-living plants. They are used in the pharmaceutical, cosmetic and food industries as active components of drugs and ingredients of artificial flavors and fragrances. α-pinene rearranges readily in the presence of an acidic catalyst to a variety of isomeric products such as tricyclene, camphene, terpinolene, limonene and others terpenes (Kirk and Othmer, 1983). The industrially most desired compound among these is camphene (Wystrach et al., 1957; Findik and Gündüz, 1997), an intermediate in the synthesis of camphor. Camphene (and others terpenes) can be produced from β-pinene too. Studies on α-pinene transformation over various types of solid catalysts towards mainly camphene production has been reported (De Stefanis et al., 1995; Severino et al., 1996; Lopez et al., 1998). Several works showing the kinetics of the liquid-phase isomerization of α-pinene has been reported (Wystrach et al., 1957; Allahverdiev et al., 1998; Allahverdiev et al., 1999), however there were not found works in the open literature that treat kinetics data of the gas-phase β- pinene isomerization. The aim of this study is to investigate the kinetics of the gas-phase β-pinene isomerization on a acid-activated bentonite clay.
II.MATERIALS AND METHODS
A natural bentonite clay (from Mendoza province, Argentine) was used as the starting material in the preparation of a catalyst. The natural sample size is < 0.074 mm. The natural clay was treated under mechanical stirring with solution of H2SO4 4 N at 90 oC for 3.5 h in a glass flask. The mass ratio of clay to the volume of acid solution was 1/10 (w/v). The obtained solids after these treatments were washed until no SO42- anions could be detected, dried at 60 oC and subsequently ground to pass through a 0.074 mm sieve. Gas-phase isomerization of β-pinene was carried out in a fixed-bed continuous flow reactor made of glass, at atmospheric pressure and loaded with 0.0028 g of catalyst. Before catalytic tests, the catalyst received a pre-treatment with nitrogen flow at 115 oC for 30 min. The catalytic reaction was investigated in the range of 58 to 90 oC. Products were analyzed by gas chomatograph (Shimadzu GC-14B instrument) with flame-ionization detector on a CBP-1 capillary column. Chemical composition of the catalyst was determined by X-ray fluorescence technique with a Philips PW 2400 spectrometer. The composition found was 73.08% SiO2, 14.70% Al2O3, 3.43% Fe2O3, 0.18% CaO, 0.71% K2O, 0.64% MgO, 0.57% Na2O, 0.42% TiO2 and 5.99% H2O (by weight). β- pinene (99%) was obtained from Fluka.
III. RESULTS AND DISCUSSION
The main isomeric products obtained of β-pinene isomerization over the acid-activated bentonite were camphene, α-pinene and limonene. These three isomers were the aim of this study. The formation of minor products were neglected. Figure 1 shows a high initial activity of catalyst, with β-pinene conversion of 100%, however the conversion shows progressive decreasing with time, reaching the stationary-state after thirteen hours of reaction. The selectivity towards camphene in the beginning of reaction is about 60%. With the decrease of β-pinene conversion with time the selectity to camphene decreases. After stationary-state the selectivity to camphene remains about of 40%.
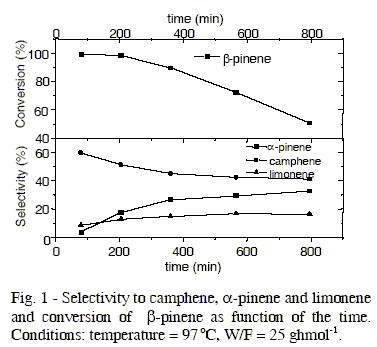
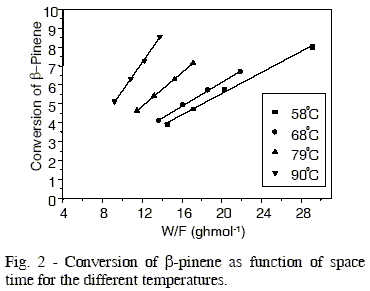
Figure 2 shows data of ß-pinene conversion as function of space time (W/F) for the different temperature. The conversion of ß-pinene was kept below 10 %, i. e., a differential reactor mode was maintained to minimize any complications which could arise from mass and heat transfer limitations. The data of conversion were obtained under stationary-state conditions. In the same figure, it is observed the linearity between ß-pinene conversion and space time, so the conversion reaction of ß-pinene can be considered as a zero order reaction.
The effect of temperature in production of different isomers is showed in Figures 3 to 6, whose values of initial reaction rate of ß-pinene conversion to products are presented in Table 1. These values were obtained from the slope of the straight lines, that correspond to the studied isomers. The rate of ß-pinene conversion (-ro) was expressed as moles of ß-pinene converted per hour per gram of catalyst, obtained from the following expression:
![]()
where Fo represents the molar flow of β-pinene fed (mol/h), Xo is the β-pinene conversion and W is the weight of catalyst.
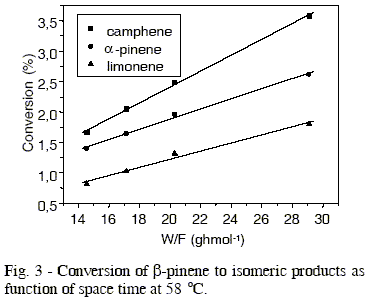
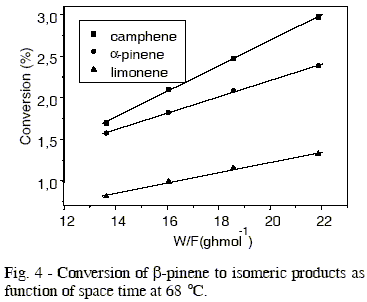
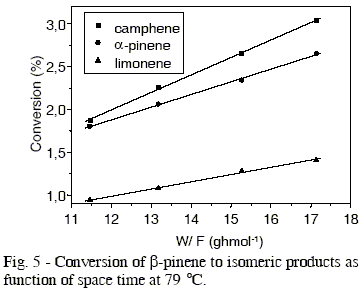
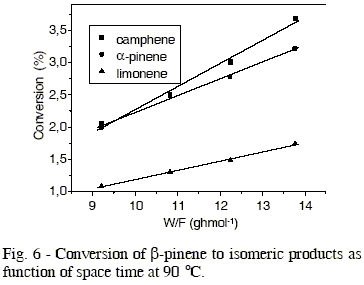
Table 1 - Reaction rate of β-pinene conversion to camphene, α-pinene e limonene in different temperatures.
Reaction rate of ß-pinene conversion as function of temperature for the different isomers is presented in Figure 7, in the form of Arrhenius diagram. The values of apparent activation energy for the formation of these isomers were determined by plotting ln (ro) vs. 1/T.
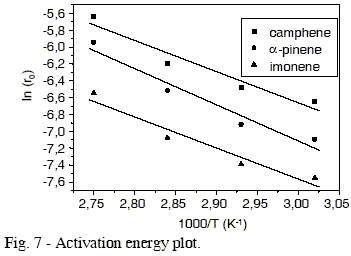
Through the analysis of Table 1 and Figure 7, we can conclude that practically there is no selectivity change with temperature. The three isomers, camphene, a- pinene and limonene showed the same value of apparent activation energy for their formation. The values of apparent activation energy for the ß-pinene conversion to a-pinene, camphene and limonene were 35.7 kJ/mol, 30.6 kJ/mol and 30.6 kJ/mol, respectively.
III. CONCLUSIONS
The kinetics of the gas-phase isomerization of ß-pinene over an acid-activated bentonite clay has been studied. The main reaction products were camphene, limonene and a-pinene. The selectivity for these products formation is practically independent on temperature. The kinetics of ß-pinene conversion was described by zeroorder kinetics with an apparent activation energy of 30- 35 kJ/mol. The activated bentonite showed a high catalytic activity for the isomerization of ß-pinene. This material domonstrated to be a efficient catalyst for the transformation of ß-pinene to camphene, with a selectivity in the beginning of reaction of about 60%, remaining in about 40% after stationary-state.
A further study intends to prepare new catalysts from the same clay material using other operational conditions in the acid activation process.
ACKNOWLEDGMENT
The authors thank to CAPES and CNPq for the financial support.
REFERENCES
1. Allahverdiev, A.I., Gündüz, G. and Murzin, D.Y., "Kinetics of alpha-pinene isomerization", Ind. Eng. Chem. Res. 37, 2373-2377 (1998).
2. Allahverdiev, A.I., Irandoust, S. and Murzin, D.Y., "Isomerization of alpha-pinene over clinoptilolite", J. Catal. 185, 352-362 (1999).
3. De Stefanis, A., Perez, G., Ursini, O. and Tomlinson, A.A.G., "PLS versus zeolites as sorbents and catalysts. II - Terpene conversions in aluminapillared clays and phosphates and medium pore zeolites", Appl. Catal. A 132, 353-365 (1995).
4. Findik, S. and Gündüz, G., "Isomerization of a-pinene to camphene", J. Am. Oil Chem. Soc. 74, 1145- 1151 (1997)
5. Kirk, R.E. and Othmer, D.F., Encyclopedia of Chemical Technology, Vol 22, 3rd Ed., John Wiley & Sons, New York, p. 709 (1983)
6. Lopez, C.M., Machado, F.J., Rodríguez, K., Méndez, B., Hasegawa, M. and Pekerar, S., "Selective liquid- phase transformation of alpha-pinene over dealuminated mordenites and Y-zeolites", Appl. Catal. A 73, 75-85 (1998).
7. Severino, A., Esculcas, A., Rocha, J., Vital , J. and Lobo, L.S., "Effect of extra-lattice aluminium species on the activity, selectivity and stability of acid zeolites in the liquid phase isomerization of alphapinene", Appl. Catal. A 142, 255-278 (1996).
8. Wystrach, V.P., Barnum, L.H. and Garber, M., J. Am. Chem. Soc. 79, 5786 (1957).
Received: May 2, 2001.
Accepted for publication: June 28, 2001.
Recommended by Subject Editor R. Gomez.












