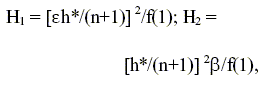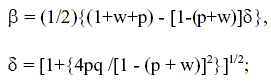Servicios Personalizados
Articulo
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. v.32 n.2 Bahía Blanca abr./jun. 2002
A parallel - Consecutive reaction scheme in three-dimensional pore structure catalyst pellet
Elio E. Gonzo
INIQUI (UNSa-CONICET), Universidad Nacional de Salta
Facultad de Ingeniería, Buenos Aires 177, (4400) Salta, Argentina
gonzo@unsa.edu.ar
Abstract — The three-dimensional pore model, that characterizes solid materials prepared by aggregating and coalescing spherical microparticles, is used to evaluate the role of structural and transport parameters in a catalytic system of parallel - consecutive first order reactions. The selection of the optimum catalyst structure (porosity), which leads to maximum reaction rates, is illustrated. The effects of intermediate product external concentration and the influence of a non-uniform catalytic activity distribution inside the pellet are also presented.
Keywords — parallel - consecutive reactions, diffusion-reaction, pore structure.
I. INTRODUCTION
A few years ago, Gottifredi et al. (1994) were able to predict the diffusional effects on selectivity and the effectiveness factor for a relatively complex parallel-consecutive reaction scheme containing a reversible step and in which all the reactions are of the first order. Also, an approximate approach that makes possible the solution for different pellet geometry and catalyst activity distributions inside the particle, and gives values extremely accurate with respect to numerical solutions, was developed.
Today, it is an essential requirement in heterogeneous catalysis to know structural pore models through which an optimum balance between diffusion and reaction can be performed. Reyes et al. (1990) and Reyes and Iglesia (1991) have developed very interesting models for the porous network and transport properties of a catalyst pellet. Particularly, the three dimensional pore structure model which results from the aggregates of spherical particles partially compressed and sintered, is a very realistic representation of a class of sol-gel derived porous materials. The sol-gel technique is well known (Stober et al., 1968; Xu and Anderson, 1991). Typically, a solution of a metal salt is converted by controlled pH precipitation into a colloidal dispersion of spherical particles of micrometric or sub-micrometric dimensions. Finally, this dispersion is compacted and sintered into pellets or extrudates.
By taking into account this porous structure model and the parallel-consecutive reaction scheme previously indicated, an optimal selection of catalyst structural properties can be performed, that leads to a maximum rate of production of the intermediate component B.
In this work it is also shown the effect of pellet porosity on selectivity and production rates, when a non-uniform activity distribution is considered.
II. ANALYSIS
The parallel-consecutive scheme here considered is
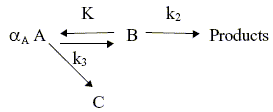
in which all the reactions are of the first order.
According to the three-dimensional pore structure model for the catalyst pellet prepared from a random aggregate of microspheres, the diffusivity factor E (Reyes and Iglesia, 1993) is a unique topological quantity, as function of the accessible porosity f, that is described by the equation:
| | (1) |
where E is the relation between the effective diffusivity and the equivalent diffusivity corresponding to the average pore radius (rp), calculated from pellet porosity and surface area per unit volume (av) as
| | (2) |
Equation (1) is valid for porosity higher than that corresponding to the percolation threshold fc, which is the minimum porosity required to have macroscopic transport (fc = 0.058).
The surface area av depends on pore size and exhibits a maximum at intermediate porosity (f » 0.5). The maximum arises from competition between creation and destruction of pore surfaces as porosity increase. A good relation functions between aV and f is (Reyes and Iglesia, 1993)
| | (3) |
The catalyst particle is considered to have either planar, cylindrical or spherical shape (n = 0, 1, 2), respectively; with characteristic length R and with an activity distribution function f(x). This function f(x) is normalized in such a way that (Gottifredi and Gonzo, 1983):
| | (4) |
where x represents the dimensionless spatial coordinate. The distribution functions, f(x) = 1 (uniform, U) and, f(x) = ax2 (non uniform, NU), were used.
The dimensionless continuity equations for A and B in an isothermal pellet can be written as:
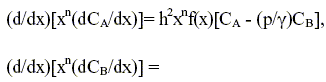 | (5) |
| | (6) |
where CA and CB are the concentrations normalized with respect to the external surface value of reactant A. The groups h2, w, p, q and γ are defined as follows
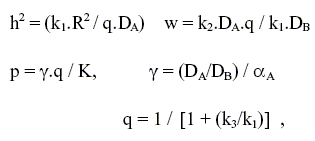 | (7) |
where D represents effective diffusivities; k1, k2 and k3 are the reaction rate coefficients and K is the equilibrium constant for the first step of the consecutive path. Since our aim is to determine the effect of pellet porosity on the reacting system, the Thiele modulus h, for the consumption of reactant A, is defined at a reference porosity (f = 0.2) and for a value equal to one.
Equations (5) and (6) must be solved subject to the following boundary conditions:
|
| (8) |
Following the work of Gottifredi et al. (1994), where the auxiliary function:
| | (9) |
and the perturbation and matching technique (Gottifredi and Gonzo,1986) were used, the effectiveness factor (η) for the consumption of A is defined as:
| | (10) |
Taking into account the pore structure model previously introduced, η can be obtained from the expression:
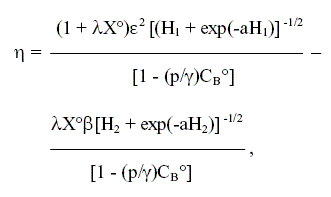 | (11) |
where:
|
| (12) |
|
| (13) |
|
| (14) |
|
| (15) |
|
| (16) |
|
| (17) |
In Eqn. (14), (Av) represents the dimensionless surface area per unit volume of the pellet and E' stands for the diffusivity factor, both normalized with respect to the corres-ponding values at an arbitrary reference porosity (f = 0.2, in this case).
As was pointed out in the previous work (Gottifredi et al. (1994)), the selectivity for the component B can be predicted from its usual definition, that is:
| | (18) |
where RB and RA are the net production rate of component B and A, respectively.
By combining Eqs. (10), (11) and (18), the production rate of component B is obtained:
| | (19) |
with
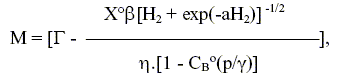
and from Eqns. (10) and (11), the rate of consumption of A may be expressed as
| | (20) |
Finally, the selectivity to component B can be predicted from Eqn. (18).
III. RESULTS AND DISCUSSION
The only structural-dependent parameters of Eqns. (18), (19) and (20) are E' and AV, both of which depend on porosity. Consequently, the value of f that maximizes the selectivity, RA or RB may be calculated from these equations.
To study the effect of the presence or absence of the parallel reaction, as well as, the diffusivities ratio and the equilibrium constant; parameters q and k3, and p, γ, w and K, respectively, were varied.
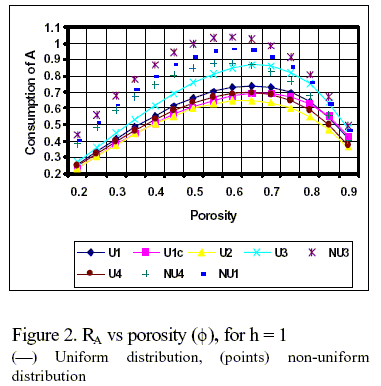
Typical results of the effect of porosity on the net rate of production of component B are illustrated in Fig.1 for n = 0 and a Thiele modulus equal to one. This intermediate value of h is far from the asymptotic solutions of the system. The selected parameters for each curve in Fig. 1 and 2 are the following:
U1: CBº = 0.1, w = 0.5, γ = 1, p =1, q = 0.6; U1c: CBº = 0.1, w = 0.5, γ = 1, p =1, q = 1 (k3 = 0); U2: CBº = 0, w = 2, γ = 1, p =10, q = 0.6; U3: CBº = 0.1, w = 2, γ = 2, p =1, q = 0.6; U4: CBº = 0.45, w = 1, γ = 2, p =1, q = 0.6; NU1: CBº = 0.1, w = 0.5, γ = 1, p =1, q = 0.6; NU3: CBº = 0.1, w = 2, γ = 2, p =1, q = 0.6; NU4: CBº = 0.45, w = 1, γ = 2, p =1, q = 0.6.
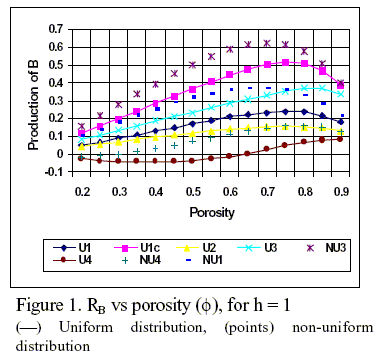
These simulations clearly show how diffusion and reaction rates compete as the catalyst structure is modified. The maximum in RB occurs at intermediate values of porosity (f = 0.65 to f = 0.85), depending on the values of the different parameters.
The effect of the parallel reaction can be seen from a comparison of U1 and U1c series, where U1c corresponds to a purely consecutive scheme (q = 1, k3 = 0). Since in U1c A is converted only in B, the net production rate of B is higher than in U1 where the parallel reaction exists (q = 0.6, k3/k1 = 2/3). Nevertheless the maximum is not affected f = 0.75 (η = 0.66 and 0.63 respectively). For the non-uniform activity distribution of the type considered here (descending towards the centre of the pellet), RB increases, as may be observed by comparing U1 and NU1. But now the maximum is affected and is obtained at f = 0.65 with η = 0.75.
Increasing the values of γ (increases the ratio DA/DB) and increasing the equilibrium constant K for the first consecutive reaction (p decreases), the net volumetric rate of B increases substantially, although the maxi-mum shifts from f = 0.75 (η = 0.51) to f = 0.85 (η = 0.84), as it is shown in curves U2 and U3.
Furthermore it should be noticed that when a non-uniform distribution is used (NU3), for the same conditions than in U3, RB increases reaching a maximum in f = 0.7 with η = 0.79, even if the effectiveness factor is lower than in U3.
The effect of external pellet concentration of the intermediate product B can be seen in curve U4 and the role of non uniform distribution of active site, for the same conditions, in NU4. It is interesting to note that for uniform distribution (U4) the rate of production of B is negative for porosity values lower than 0.63, which means that B is consumed instead to being produced. Nevertheless, for an activity distribution decreasing toward the pellet centre (NU4), RB increases and reaches a maximum at f = 0.8 with η =0.873. Also, the porosity where RB = 0 is smaller than for uniform distribution (f = 0.3 with η = 0.576).
In Fig.2, it is depicted the variation of RA with f for the conditions previously indicated. As expected, the net rate of consumption of A is always positive, even for the cases U4 and NU4. The maxima are reached at lower values of f than those corresponding to RB , under the same conditions.
The effectiveness factor η and the selectivity S increase constantly with f for all cases studied.
IV. CONCLUSIONS
A method for evaluating the porosity effect on the net production rate of intermediate product B and reactant A is proposed. Furthermore the importance of selecting the optimum value of the structural parameter to obtain maximum rates in complex reaction system is highlighted. The present approach provides a clear understanding of the role of diffusion and reaction inside a catalyst pellet.
The effect of reactant and product concentrations and the influence of a non-uniform catalyst distribution on the optimal porosity that maximizes the rate RA or RB, is clearly shown. The maximum rates occur at intermediate values of porosity (0.65 <f< 0.85) and in a diffusionlimited regime. The effectiveness factor for the total consumption of A is in the range [0.51 - 0.87].
REFERENCES
1. Gottifredi, J., E. Gonzo and G. Froment, "Diffusion and reaction inside a catalyst pellet for a parallel-consecutive reaction scheme," Chem. Engng. Sci. 49, 2399- 2403 (1994).
2. Gottifredi, J. and E. Gonzo, "Application of perturbation and matching techniques to solve transport phenomena pro-blems," in Advances in Transport Processes, Volume IV, Mujumdar and Mashelkar Eds., Chapter VII, Wiley Eastern, New Delhi, (1986).
3. Gottifredi, J. and E. Gonzo, Rational approximations of effectiveness factor and general criteria for heat and mass transport limitations, "Catal. Rev.-Sci Eng., 25, 119-140 (1983).
4. Reyes, S. and E. Iglesia, "Effective diffusivities in catalyst pellets : New model porous structures and transport simulation techniques," J. of Catal. 129, 457 -472 (1991).
5. Reyes, S. and E. Iglesia, "Simulation techniques for the characterization of structural and transport properties of catalyst pellets," in Computer - Aided Design of Catalysts, Becker, E. and C. Pereira, Eds., Chapter 5, Marcel Dekker Inc., New York (1993).
6. Reyes, S., E. Iglesia and Y. Chiew, "Monte Carlo simulations of effective diffusivities in three-dimensional pore structures," Proc. Mater. Res. Soc., 195, 553 -558 (1990).
7. Stober, W., A. Fink and E. Bohn, "Controlled growth of monodisperse silica spheres in the micron size range," J. Colloid Inter. Sci. 26, 62-69 (1968).
8. Xu, Q. and M. Anderson, "Synthesis of porosity controlled ceramic membranes," J. Mater. Res. 6, 1073-1079 (1991).
Received: April 5, 2000.
Accepted for publication: July 9, 2001.
Recommended by Subject Editor P. Toledo.