Servicios Personalizados
Articulo
Latin American applied research
versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.42 no.1 Bahía Blanca ene. 2012
A Simple model for cholesterol accumulation on the artery wall near stagnation points
V.C. Gessagui†, D. Tanoni‡, C.A. Perazzo* and A. E. Larreteguy‡
†Facultad de Ingeniería, Universidad Nacional de La Pampa, La Pampa, Argentina
gessaghi@ing.unlpam.edu.ar
‡Instituto de Tecnologia, Facultad de Ingeniería y Ciencias Exactas,
Universidad Argentina de la Empresa, Buenos Aires, Argentina
*Dto. de Física y Química, Universidad Favaloro and CONICET, Buenos Aires, Argentina
Abstract— Cardiovascular diseases are one of the leading causes of death in the first world countries nowadays and atherosclerosis is the most relevant among them. It is a disease that affects medium and large size arteries, which causes the formation of plaques within the artery wall. These plaques, called atheromas, develop due to the accumulation of fat, cholesterol, cell debris, smooth muscle cells and other cells and substances. Atheromas may cause temporary or definitive lack of blood supply to organs, such as the heart or the brain.
This article proposes a model for cholesterol accumulation and fatty streak formation, which are possible precursors of atheroma. The model is basically a mass balance of low density lipoproteins (LDL) in the intima. The inflow, outflow, oxidation, and consumption of LDL is modeled combining partial models and endothelial LDL permeability correlations available in the literature.
A simple zero-dimensional case was run for assessing the sensibility of the model to the initial conditions. A more complex case of a two-dimensional flow in the vecinity of a stagnation point on a rigid wall was used for evaluating the influence of spatial variations of the wall-shear stresses. Blood flow was assumed as an steady flow of an homogeneous newtonian fluid, while blood pressure and LDL blood concentration values were assumed as physiologic.
Results showing local LDL mass accumulation and intimal thickening evolution for the first case, and spatial distribution of the initial intimal growth rate for the second one, indicate that there is a very short initial transient behaviour of LDL mass accumulation and intimal thickeness, which may well be considered instantaneous compared to the usual periods involved in the lesion formation. This allows the use of simple quasi-steady solution in future computational implementations of more realistic applications involving 3D arterial geometries with wall remodelling, that will significantly reduce the computational effort.
Keywords—Atherosclerosis; Hemodynamics; Cholesterol Transport
I. INTRODUCTION
Atherosclerosis is an inflammatory disease that affects large and medium-sized arteries Ross (1999). The atherosclerotic lesion, called atheroma, is a focal thickening of the innermost layer of the artery wall, or intima. These lesions consist mainly of accumulation of lipids, endothelial and vascular smooth muscle cells (SMC), connective tissue and debris (Berliner et al., 1995). Atheromas may cause a temporary or permanent lack of blood supply to some organ and is the leading cause of death in developed countries (American Heart Association Statistic Commitee and Stroke Statistics Subcommitee. 2007). This is the reason why there has been a big effort devoted to learn and understand its genesis and to find the main risk factors for this disease (Altman, 2003; Hinderliter and Caughey,2003).
Atheromas usually develop in arterial bifurcations or regions with marked curvature. Four decades ago, it was proposed that forces exerted by the blood flow on the artery wall have a major influence on the location where these lesions appear (Fry, 1969; Caro et al., 1971). Nowadays, researchers agree that atheromas develop in areas with complex flow patterns, such as recirculation and/or secondary flows, where the endothelium is subjected to low and oscillating shear stresses, which are thought to be the cause of the location of the plaques (Friedman and Giddens, 2005; Ku, 1997). It has been proposed that these low and oscillating stresses may alter the permeability of the endothelium (Ogunrinade et al., 2002; Himburg et al., 2004) favoring extracellular low density lipoproteins (LDL) accumulation and progressive oxidation in these locations.
Another important event in the initiation of the atheroma is leukocyte recruitment. Mono-cytes and T-lymphocytes tend to accumulate in the early atherosclerotic lesion. The monocytes become macrophages in the artery wall and ingest the oxidized LDL (LDLox) turning into foam cells, commonly found in atheromas (Braunwald et al., 2008). The evolution of the atheroma into a more complex lesion involves SMC migration and proliferation as well as accumulation of other molecules and substances. It is the extracellular matrix rather than the cells themselves that makes up much of the volume of an advanced atherosclerotic plaque, through the accumulation of extracellular matrix macromolecules, such as collagen and proteoglycans produced by the SMCs (Braunwald et al., 2008).
Despite all these processes that occur, some researchers believe that the oxidation of the accumulated LDL is the factor that initiates plaque formation (Holtzman, 2008). This is why, lately, some researchers simulated coupled transport of LDL both in the lumen and through the artery wall (Stangeby and Ethier, 2002; Sun et al., 2006). These works intended to predict the variation of the transendothelial LDL flux along the axial direction of an artery with stenosis, as well as the LDL concentration in the wall.
Other recent works found in literature proposed more complete mathematical models of the early stages of this desease. Ibragimov et al. (2005, 2007, 2010) proposed and studied the evolution of an initial PDE model for the inflammatory response in early lesion formation, which includes detail models of accumulation of debris, immune cells, smooth muscle cells and other processes involved in this response in a fixed domain. Their work focuses on the spatial characteristics on the through the addition of a diffusion term and they describe the atherogenesis as the result of an instability in the configuration of the intima. Other researchers proposed models that focused on the time dependence of the inflammatory process. Ougrinovskaia et al. (2010) proposed a simplified ODE model for this purpose and they found that it is macrophage proliferation and constant signaling to the endothelial cells, rather than an increasing influx of modified LDL, that drives lesion instability.
We present here a much simpler model for predicting fatty streaks formation due to accumulation of oxidized LDL and other non fat substances within the artery wall, which are possible precursors of atheroma. Our model captures the localization effects of the hemodynamic forces, by modeling the wall permeability as dependent of the wall shear stress. This model includes the initiating phenomena to which other phenomena can be added to reach a more complete model. The model proposed has been implemented and analysed for a single position with two different initial conditions and in a simple 2D geometry of a stagnation point on a rigid wall.
II. THE MODEL
A. The system of equations
The model comprises two main variables that depend on space and time, namely the intimal LDL mass accumulation per unit surface area, m, and the intimal thickness, e. Their time evolution result from solving the following system of two coupled first order ordinary differential equations
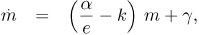 | (1) |
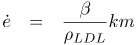 | (2) |
where k, β and ρLDL are constants and where the parameters α and γ are functions of the physiologic transport properties of the endothelium, the intima, the media and the physiologic consumption of the intimal cells.
The details of the derivation of these two equations are explained in the next subsections.
It should be noted that Eqs.(l) and (2), along with proper initial conditions for m and e, describe an initial value problem on these two variables.
For reasons that will be clarified in the next section, it is also important to note that for 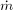 tending to zero and for constant values of k, α and γ, Eq. (1) has an quasi-steady solution given by
tending to zero and for constant values of k, α and γ, Eq. (1) has an quasi-steady solution given by
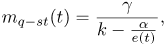 | (3) |
noting that this solution will converge to since e(t) converges to infinity as t converges to infinity
B. Intimal growth rate equation
The rate of change of the intimal thickness or intimal growth rate  , expressed by Eq. (2), is modeled as proportional to the rate of accumulation of LDLox mass per unit of lumen-intima interphase area
, expressed by Eq. (2), is modeled as proportional to the rate of accumulation of LDLox mass per unit of lumen-intima interphase area 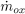 , as expressed in the following equation:
, as expressed in the following equation:
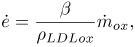 | (4) |
where β is a constant to be explained later.
Four major assumptions are made here, the first one being that the change in volume of the intima is caused only due to the accumulation of LDLox and the LDLox density relates the mass accumulated to the volume changes. These assumption is based in the fact that in early lesions lipoprotein particles appear to decorate the proteoglycan of the arterial intima, abandoning the solution state and coalescing into aggregates bounded to the extracelular matrix, having increased susceptibility to oxidative or other chemical modifications (Braunwald et al., 2008).
The second and third assumptions are that the density of LDLox is not modified when bounded to macrophage and that is equal to the density of LDL, which results similar to the assumption made by Calvez et al. (2009), of a constant maximum cellular density.
Finally, the fourth assumption is that local relative changes in interphase area are negligible compared to local relative changes in thickness.
Constant β is intended to consider the contribution of additional accumulation of non-lipidic substances. A study made by Guyton and Klemp (1993) found that in lesions obtained from people that died from causes other than atherosclerosis, approximately 50 % of the total intimal thickening of post-mortem human aortic fatty streaks was due to lipid accumulation and the rest to collagen and other non-lipidic substances. We set then β = 2 to account for this additional non-lipidic accumulation.
To complete the derivation of Eq. 2, the rate of change of mass of LDLox inside the intima, 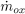 , is evaluated assuming that all LDL that undergoes oxidation accumulates in the intima bounded to a macrophage cell. As Cobbold et al. (2002) modeled for the in vitro case the oxidation process of LDL actually takes more than one step,. They modeled each reaction as a linear reaction with a free radical with no spatial dependence. Later Ibragimov et al. (2005) modified this equations to include a spatial dependent diffusion term, considering only one-step reaction process with no antioxidant factor or HDL and constant concentration of free radicals. In order to focus on the influence of the influence of hemodynamic forces specially by letting the boundary to freely move, we decided to use an even more simplified model considering a one-step irreversible first order reaction, where the concentration of oxidative agents is much larger than the concentration of LDL, and not allowing for mitigation of the oxidation process or the potential removal of LDLox, that would occur in the presence of antioxidants or HDL molecules. This means that
, is evaluated assuming that all LDL that undergoes oxidation accumulates in the intima bounded to a macrophage cell. As Cobbold et al. (2002) modeled for the in vitro case the oxidation process of LDL actually takes more than one step,. They modeled each reaction as a linear reaction with a free radical with no spatial dependence. Later Ibragimov et al. (2005) modified this equations to include a spatial dependent diffusion term, considering only one-step reaction process with no antioxidant factor or HDL and constant concentration of free radicals. In order to focus on the influence of the influence of hemodynamic forces specially by letting the boundary to freely move, we decided to use an even more simplified model considering a one-step irreversible first order reaction, where the concentration of oxidative agents is much larger than the concentration of LDL, and not allowing for mitigation of the oxidation process or the potential removal of LDLox, that would occur in the presence of antioxidants or HDL molecules. This means that 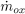 results proportional to the mass of LDL in the intima, m, as expressed in the following equation:
results proportional to the mass of LDL in the intima, m, as expressed in the following equation:
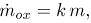 | (5) |
where k is the oxidation rate. Replacing this expression in Eq. (4) we get the second equation of the model, namely Eq. (2).
C. LDL mass accumulation rate equation
The rate of change of the mass of LDL inside the intima, represented by Eq. (1), is calculated as (see Fig. 1)
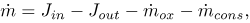 | (6) |
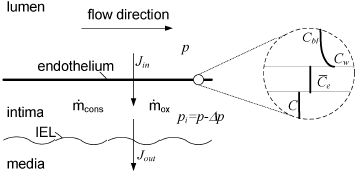
Figure 1: Schematic illustration of the intimal growth model; the circle on the right shows a closer look at endothelium indicating LDL concentrations at every location. IEL: Internal Elastic Lamina
where Jinand Jout are the fluxes of LDL entering and leaving the intima respectively, and 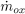 and
and 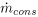 are the rates of consumption of LDL by oxidation and by physiologic ingestion of the wall cells per unit area, respectively. All other reactions are neglected and all the fluxes through the arterial wall are assumed to occur only in the radial direction.
are the rates of consumption of LDL by oxidation and by physiologic ingestion of the wall cells per unit area, respectively. All other reactions are neglected and all the fluxes through the arterial wall are assumed to occur only in the radial direction.
The first term, Jin is evaluated as follows. The flux of LDL goes through the endothelium and into the intima by two different known pathways, one being the vesicular transport and the other the transient or leaky junctions. On the other side, the flux of water and other hydrophilic solutes (Tarbell, 2003; Michel and Curry, 1999) have intercellular junctions as their main pathway. It has been measured, however, that vesicular transport accounts for a scarce 9% of the total LDL transport to the intima (Cancel et al., 2007). Therefore, we model solute plasma transendothelial transport, following Sun et al. (2006) and Yang and Vafai (2006, 2008), by means of the Kedem-Katchalsky equations:
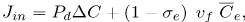 | (7) |
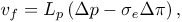 | (8) |
where vf is the filtration velocity; Lp, Pd and σe are the transport parameters of the endothelial membrane regulating plasma and LDL flux into the intima. Defining C and Cw as the LDL concentration within the intima and at the lumen-wall interphase respectively, ΔC, Δp and 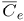 are defined as
are defined as
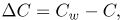 | (9) |
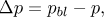 | (10) |
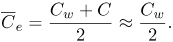 | (11) |
To estimate Cw we used a simple one-dimensional model that balances convective and diffusive transport within the boundary layer (BL), assuming a uniform known concentration outside the BL and a uniform blood pressure. This estimation is valid because there are no reactions occurring in the lumen that can cause LDL to appear or disappear, and because the mass transport BL on the lumen side is very thin compared to the velocity boundary layer (see Tarbell, 2003). These assumptions lead to an exponential solution for the radial distribution of the LDL concentration in the BL. When solving explicitly for Cw it results:
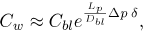 | (12) |
where Dbl is the diffusion coefficient for LDL in blood and δ is the hydrodynamic BL thickness assumed constant based on the Graetz-Nusselt solution for a cylinder of smooth rigid walls with dimensions similar to the common carotid artery (Stangeby and Ethier, 2002) (see table 1).
Table 1: Parameter values used in the model
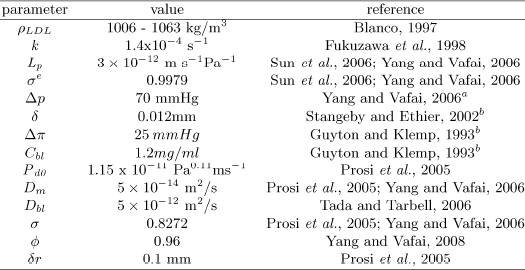
aPhysiologic values
bBased on Graez-Nusselt solution for a cylinder
Equations (7) and (8) reflect that the factors that control LDL and water flux across the endothelium are the transmembrane hydrodynamic and osmotic pressure differences and the LDL concentration difference. The osmotic pressure difference Δπ is caused by the presence of many different molecules in the plasma, namely salts, proteins, lipoproteins, etc. The total osmotic pressure difference between plasma and interstitial fluids in the capillaries at body temperature was assumed constant as suggested by others (Tarbell, 2003; Hodgson and Tarbell, 2002) (see table 1).
While Lp and σe were assumed constant in the present work (see table 1), the endothelial diffusive permeability Pd is modelled as a function of the wall shear stress τw, as said in the previous section. Although it is not clear how endothelial cells sense τw, there are a few empirical models in literature that correlate permeability to macromolecules with different hemodynamic parameters (Rappitsch and Perktold, 1996; Friedman and Fry, 1993; Himburg et al., 2004; LaMack et al., 2005). Following Himburg et al. (2004), the shear-dependent permeability is modelled as
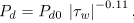 | (13) |
where Pd0 is constant scaled so that the permeability is 2 x 10-10m s-1 in the straight portions of the artery, which is considered a normal or reference value in the literature (see table 1).
The second term of Eq. (6), Jout, was calculated as the sum of a diffusive and a convective flux, Jdif f and Jconv, defined as
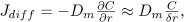 | (14) |
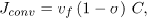 | (15) |
where the Dm and σ were assumed constant (see table 1). Prosi et al. (2005) showed that both the value and the gradient of LDL concentration within the intima are not sensible to the conditions assumed at the media. In the present model, we assumed that the concentration in the media is negligible at a distance δr from the intima-media interphase, and thus Jconv is simply evaluated as proportional to vf and C.
The last term,  , represents a sink due to physiologic LDL consumption by the wall cells. Not surprisingly, the results obtained with the model without this sink would predict accumulation of LDL even in regions where it is unlikely to occur under physiologic conditions, i. e., in the straight portions of the arteries. To tackle this problem in a simple manner, the physiologic cell consumption is adjusted in each particular problem to be:
, represents a sink due to physiologic LDL consumption by the wall cells. Not surprisingly, the results obtained with the model without this sink would predict accumulation of LDL even in regions where it is unlikely to occur under physiologic conditions, i. e., in the straight portions of the arteries. To tackle this problem in a simple manner, the physiologic cell consumption is adjusted in each particular problem to be:
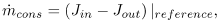 | (16) |
where the reference conditions correspond to the physiologic conditions for Δp and Cbl (see Table 1), and to a value of τw corresponding to straight zones of the flow domain in which there is experimental evidence that there is no significant growth under normal physiologic conditions. For example, straight portions of the carotid artery have values of τw higher than 0.5Pa. It is important to note, however, that this particular value may be more general than simply an example value, because it is also cited by other authors as a threshold value, since lower wall shear stresses are associated with reduction in several vascular wall functions such as endothelial nitric oxide syntheses production, vasodilatation and endothelial cell repair (Cunningham and Gotlieb, 2005).
Finally, to close the model is it necessary to relate LDL mass to LDL concentration in the intima. Consider a portion of volume V of the intima, defined by a patch of area A and the full thickness e of the intima, so that 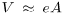 . Assuming the intima as a porous medium filled completely with plasma, the volume of the intima effectively occupied by plasma can be expressed as Vpl = V
. Assuming the intima as a porous medium filled completely with plasma, the volume of the intima effectively occupied by plasma can be expressed as Vpl = V , where
, where  is the porosity of the intima, assumed constant. Recalling that m is defined as LDL mass per unit area, the concentration may then be expressed as C = m A/Vpl, which for small e results finally in
is the porosity of the intima, assumed constant. Recalling that m is defined as LDL mass per unit area, the concentration may then be expressed as C = m A/Vpl, which for small e results finally in
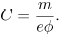 | (17) |
With all these assumptions Eq. (6) can be rewritten as Eq. (1), where
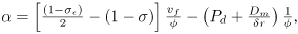 | (18) |
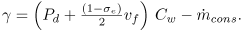 | (19) |
It should be noted that all the parameters in the model are constant in space and time, except for the endothelial permeability that for a given time varies with space through its dependence on the wall shear stress distribution, which in turn varies also in time following the changes in the flow pattern due to remodelling of the arterial geometry. As a consequence, the rate of LDL mass accumulation varies both in space and in time.
Table 1 shows the values adopted in the model for all the parameters, together with the reference to the literature where the value was taken from.
It is important to note that, based on the conventional histological classification (Stary, 2000), the model developed here is valid only for lesions on its early stages since in more advanced stages other processes such as inflammation and plaque rupture become relevant. However, there is no exact measure in time of when these stages ends and the lesion evolves to the next one.
III. METHODS
As already mentioned, the growth model presented in the previous section comprises two coupled ordinary differential equations, namely Eqs. (1) and (2), that need to be solved together.
To better understand the intimal growth rate model behavior and predictions, we used the model to compute the initial growth rate in some simple situations. For initial growth rate we mean the rate before any significant remodellation of the surface modifies the geometry and consequently the flow itself.
For this purpouse we solved the differential equations numerically, aproximating the time derivative by a conventional Euler scheme, with a time step of 1 second, more than enough to consider the solution "exact", using a simple calculus spreadsheet.
First, in what we call case (a), we consider a single location on the endothelium, fixed conditions, and a fixed wall shear stress value τw = 0.01Pa, commonly found in regions of lesion formation in the arterial system. We analyzed LDL mass accumulation and intimal thickness as a function of time, starting from two different initial values of m and a unique initial value of e equal to the value of a normal intima, e0 = 50μm. We also studied the evolution of m with time for the same τw and e0, for an initial condition m0 = 0mg/m2, for four different conditions of Cbl and Δp.
In case (b), and to further explore the results of the model regarding τw distribution, we brought into consideration the fact that τw is in general not uniform. In the arterial tree, even with pressure and blood cholesterol kept constant, the spatial variation of τw causes the intimal growth rate to depend on space. To study a simple but also relevant case of variation of τw we considered the prediction of the initial intimal growth rate in a two-dimensional flow in the vicinity of a stagnation point on a rigid surface, as shown in Fig. 2. This kind of flow patterns occur in, for example, reat-tachment points downstream of flow recirculations, locations well known as candidates for the growth of atheromas.
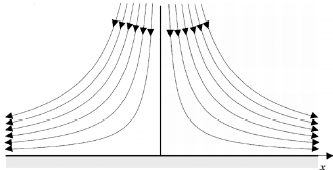
Figure 2: Schematic drawing of a two dimensional flow in the vecinity of a stagnation point on a rigid surface.
This simple case has an analytical solution for the flow (Batchelor, 2002),, showing a linear dependence of τw as a function of the position x along the wall, with x = 0 corresponding to the stagnation point itself. Assuming typical velocities found in parts of the arterial system, of about 0.2m/s far from the wall, and using viscosity and density values from normal blood (see table 1), the analytical solution predicts that τw will change at 12 Pa / m away from the stagnation point.
IV. RESULTS
In reference to case (a), Figures 3(a) and (b) show the variation of m and e with time for the first case described in the previous section. As shown in Fig. 3(a), for two different initial mass values used, m reaches the quasi-steady solution mq−st predicted by Eq. (3) in less than six hours. Figure 3(b) shows that after a very short transient that is different for different initial conditions, the thickness of the intima e increases approximately linearly with time and the slope is the same for both cases. For example, a difference of 5mg/m2 in the initial mass causes a difference of 0.005μm in the thicknessat any given time after the initial transient.
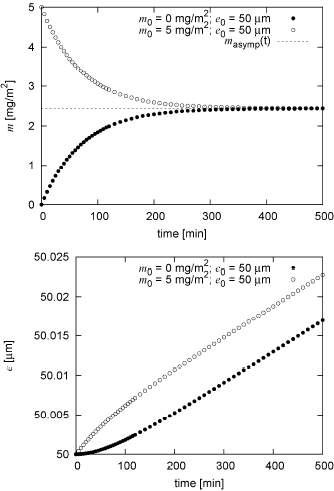
Figure 3: (a) Variation of m with time for τw = 0.01 Pa, for physiologic conditions, for two different initial conditions for m and the same initial condition for e, compared to the quasi-steady solution mq-st.
(b) Variation of e with time for the same conditions.
Figure 4 shows the behaviour of m for different values of Cbl and Δp. The higher the Cbl, the higher the LDL mass per unit area that accumulates within de intima. It is also important to note that, although higher values of Δp and Cbl seem to cause m to reach mq−st faster, the variation is not significant and in all cases as in the order of hours.
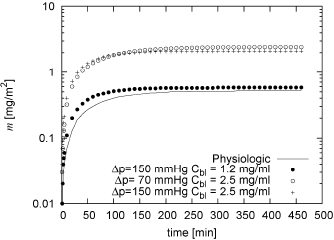
Figure 4: Variation of m with time for τw = 0.01 Pa, for initial conditions , for physiologic conditions of Cbl and Δp (see table 1) and for three other combinations of Cbl and Δp
Regarding case (b), Fig. 5 shows three different curves. The solid line shows the spatial distribution of Pd using the dependence on τw described by Eq. (13), and the variation of τw with x as described the previous section for the flow in the vecinity of a stagnation point. The filled doted curve shows the model prediction of the initial spatial distribution of  assuming there is no consumption term
assuming there is no consumption term  and the hollow doted curve shows the same variable but bwith
and the hollow doted curve shows the same variable but bwith  computed as described in Eq. (16). As evident if this term is not present the model would predict gorowth everywhere inn this geometry, while the inclusion of
computed as described in Eq. (16). As evident if this term is not present the model would predict gorowth everywhere inn this geometry, while the inclusion of  diminishes the growth rate, and would even predict negative growth rates for regions with τw > τw (reference) if it were not limited to non-negative values.
diminishes the growth rate, and would even predict negative growth rates for regions with τw > τw (reference) if it were not limited to non-negative values.
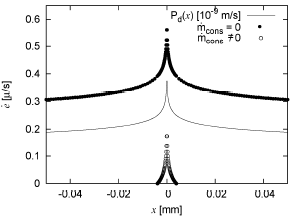
Figure 5: Initial  predicted by the model around a stagnation point at x=0.
predicted by the model around a stagnation point at x=0.
V. DISCUSSION
Results in Fig. 3(a) show that m reaches the quasi-steady solution mq−st in a few hours, indicating that this process may well be considered instantaneous compared to the usual periods involved in the lesion formation (i.e. decades). In other words, the LDL mass accumulated in the intima reacts almost instantaneously to any changes in the parameters that control the mass balance in the intima, quickly reaching its new quasi-steady solution.
Figure 3(b) shows that, for a given τw and for constant Cbl and Δp, the fact that LDL mass accumulated in the intima settles almost immediately to the quasi-steady solution, causes the rate of accumulation of LDLox mass and therefore  to quickly become approximately constant in time. Consequently, after a very short transient, the thickness of the intima e increases approximately linearly with a slope that is independent of m0.
to quickly become approximately constant in time. Consequently, after a very short transient, the thickness of the intima e increases approximately linearly with a slope that is independent of m0.
When different conditions of Δp and Cbl are analysed, see Fig. 4, the model predicts that an increase of Δp does not influence the accumulation of LDL as much as Cbl does. The reason for this is that an increase in Δp implies an increment in the flux of plasma going into and out of the intima, consequently, not only the convective incoming flux of LDL increases, but also the outgoing flux of LDL does. On the other hand, the higher the Cbl, the higher the diffusive incoming flux, resulting in more LDL mass per unit area accumulating within de intima.
Regarding Figure 5, the first thing to note is that there is also a significant correlation between  and Pd. Since the diffusive incoming LDL flux is the only one that depends on this parameter, this correlation shows the relevance of this flux over the convective flux to define LDL accumulation, which becomes specially important in regions of low wall shear stress. The effect of substracting
and Pd. Since the diffusive incoming LDL flux is the only one that depends on this parameter, this correlation shows the relevance of this flux over the convective flux to define LDL accumulation, which becomes specially important in regions of low wall shear stress. The effect of substracting 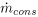 is evident in the figure, and it is important to be aware that although this parameter was added to the model and adjusted to prevent unrealistic growing for certain physiologic conditions and for regions with τw > τw(reference), it is clearly affecting the growth everywhere else.
is evident in the figure, and it is important to be aware that although this parameter was added to the model and adjusted to prevent unrealistic growing for certain physiologic conditions and for regions with τw > τw(reference), it is clearly affecting the growth everywhere else.
VI. CONCLUSIONS
Fatty streak formation and intimal thickening is modelled as a function of the accumulation of LDLox in the intima. The model is based on a balance of LDL mass in the intima along with relations for calculating the rate of LDL oxidation and the corresponding rate of increase of the intimal thickness.
The basics of the model behavior were analyzed in simple situations, such as a local analysis with fixed wall shear stress value and constant parameters. From this case it was concluded that the initial transient behaviour of LDL mass accumulation and intimal thickness is very short indicating that this process may well be considered instantaneous compared to the usual periods involved in the lesion formation (i.e. decades). This conclusion makes it possible to skip the detailed solution of Eq. (1) and directly use the quasi-steady one in future implementations of more realistic applications involving 3D arterial geometries with wall remodelling. The fact that intimal thickness grows linearly with time after this short transient will definitely reduce the computational effort for this future implementations.
The model seems to represent qualitatively well the response to changes in hemodynamic pressure and blood cholesterol concentration. Keeping in mind that these two variables are known to be major risk factors for the disease under study, especially the latter, our model does predict a greater accumulation of LDL when these variables are increased, and is more significant for the blood cholesterol concentration.
The other case analyzed here, which corresponds to a simple two-dimensional flow in the vicinity of a stagnation point, showed that the model predicts a peak for the initial growth rate for very low values of wall shear stresses, which fades away with distance. In this aspect the model seems to include the most fundamental processes since it reproduces adequately some qualitative aspects of the phenomenon, namely, that the thickness of the wall increases significantly in a few small areas, and that these areas are located in regions of complex flow patterns that cause low wall shear stresses.
In order to plan for future developments, some processes that could be included to have a more realistic idea of the evolution of the early lesions are, for example, macrophage migration and accumulation, changes in endothelial permeability caused by cell apoptosis due to hypoxia, or the effect of high density lipoproteins causing a reverse LDL flow and reducing the LDLox concentration within the intima.
A much more challenging task would be to include in this model factors such as SMC migration and reproduction, inflammatory effects which causes lots of T-cells and monocytes to migrate and segregate substances that will attract more inflammatory cells to the wall, and influence the endothelial permeability. This has been done by others but for a fixed permeability value without spacial dependence or with very little dependence through a diffusion term. Modelling advanced lesion stages, not only requires accounting for the accumulation of a great variety of substances but also the modelling of other complex phenomena such as cup erosion or rupture and thrombosis maintaining the spatial dependence.
The analysis presented here provides the basics to perform a simple but realistic implementation to simulate plaque growth in 3D remodelling realistic arterial geometries, not requiring the solution of complex systems of differential equations.
Nomenclature
| A | lumen-intima interphase area |
| BC | Boundary conditions |
| BL | boundary layer |
| C | concentration of LDL within the intima |
| Cbl | bulk concentration of LDL in blood |
| Cw | LDL concentration at the lumen-wall interphase |
| ΔC | transendothelial LDL concentration difference |
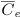 | mean endothelial concentration |
| CCA | common carotid artery |
| Dbl | diffusion coefficient for LDL in blood |
| Dm | diffusion coefficient for LDL in the media |
| EC | external carotid artery |
| e | intimal thickness |
| FD | flow divider |
| FVM | Finite Volume Method |
| IC | internal carotid artery |
| IEL | internal elastic lamina |
| Jin | total flux of LDL going into the intima |
| Jout | total flux of LDL going out of the intima |
| Jdiff | diffusive flux of LDL going out of the intima |
| Jconv | convective flux of LDL going out of the intima |
| k | reaction rate of LDL oxidation |
| LDL | Low density lipoprotein |
| LDLox | oxidized LDL |
| Lp | endothelial hydraulic conductivity |
| m | mass of LDL within the intima per unit area |
| mq−st | quasi-steady solution for m |
| mox | mass of LDLox within the intima per unit area |
 | physiologic rate of consumption of LDL in the intima per unit area |
| pbl | blood pressure |
| Pd | endothelial diffusive permeability |
| Pd0 | scaling constant |
| p | subendothelial hydrodynamic pressure |
| Δp | transendothelial hydrodynamic pressure difference |
| Δπ | transmembrane osmotic pressure difference |
| vf | plasma filtration velocity |
| V | total volume of the intima |
| Vpl | volume of the intima occupied by plasma Greek symbols |
| α | parameter resulting from the mass balance |
| β | constant in the intimal growth rate equation |
| γ | parameter resulting from the mass balance |
| δ | hydrodynamic boundary layer thickness |
| δr | distance from IEL to boundary |
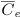 | density of LDL |
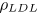 | density of LDLox |
| σe | endothelial reflection coefficient |
| σ | osmotic intimal reflection coefficient |
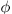 | porosity of the intima |
VII. ACKNOWLEDGMENTS
The authors wish to thank the support of Agencia Nacional de Promocion Científica y Tecnológica of Argentina through the Program for Technological Modernization, BID 1728/OC-AR, grant PICTO-CRUP 31384. VCG thanks the support of Facultad de Ingeniería, UNLPam. CAP thanks grants PICTO 21360.
REFERENCES
1. Altman, R., "Risk Factors in Coronary Atherosclerosis Athero-Inflammation: The Meeting Point," Thrombosis Journal, 1, 4 (2003). 2. American Heart Association Statistic Commitee and Stroke Statistics Subcommi-tee, Heart diseases and stroke statistics - 2007 Update At-a-Glance, American Heart Association and American Stroke Association (2007).
3. Batchelor, G.K., An introduction to fluid dynamics, Cambridge University Press (2002).
4. Berliner, J.A., M. Navab, A.M. Fogelman, J.S. Frank, L.L. Demer, P.A. Edwards, A.D. Watson and A.J. Lusis, "Atherosclerosis: Basic Mechanisms : Oxidation, Inflammation, and Genetics," Circulation, 91, 2488-2496 (1995).
5. Blanco, A., Química biológica, Sixth Ed., El Ateneo, Buenos Aires (1997).
6. Braunwald, E., D.P. Ziper and P. Libby, "The vascular biology of atherosclerosis," Heart disease: A textbook of cardiovascular medicine, Ed. W.B.C.O. Saunders, Eighth Edition, Chapter 38 (2008)
7. Caro, C.G., J.M. Fitz-Gerald and R.C. Schroter, "Atheroma and arterial wall shear. Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis," Proceedings of the Royal Society of London. Series B: Biological Sciences, 177, 109-159 (1971).
8. Calvez, V., A. Ebde, N. Meunier and A. Raoult, "Mathematical modelling of the atherosclerotic plaque formation," Proceedings of the Royal Society of London. Series B: Biological Sciences, 28, 1-12 (2009).
9. Cancel, L.M., A. Fitting and J.M. Tarbell, "In vitro study of LDL transport under pressurized (convective) conditions,"Am. J. Physiol. Heart Circ- Physiol., 293, H123-132 (2007).
10. Cobbold, C.A., J.A. Sherratt and S.R.J. Maxwell, "Lipoprotein oxidation and ints significance for atherosclerosis: a mathematical approach," Bulletin of Mathematical Biology, 64, 65-95 (2002).
11. Cunningham, K.S. and A.I. Gotlieb, "The role of shear stress in the pathogenesis of atherosclerosis," Lavoratory Investigation, 85, 9-23 (2005).
12. Friedman, M.H. and D.L. Fry, "Arterial permeability dynamics and vascular disease," Atherosclerosis, 104, 189-194 (1993).
13. Friedman, M.H. and D.P. Giddens, "Blood Flow in Major Blood Vessels: Modeling and Experiments," Annals of Biomedical Engineering, 33, 1710-1713 (2005).
14. Fry, D.L., "Certain histological and chemical responses of the vascular interface to acutely induced mechanical stress in the aorta of the dog," Circulation Research, 24, 93-108 (1969).
15. Fukuzawa, K., Y. Inokami, A. Tokumura, J. Terao and A. Suzuki, "Rate constants for quenching singlet oxygen and activities for inhibiting lipid peroxidation of carotenoids and a-tocopherol in liposomes," Lipids, 33, 751-756 (1998).
16. Guyton, J.R. and K.F. Klemp, "Transitional features in human atherosclerosis. Intimal thickening, cholesterol clefts, and cell loss in human aortic fatty streaks," American Journal of Pathology, 143, 1444-1457 (1993).
17. Himburg, H.A., D.M. Grzybowski, A.L. Hazel, J.A. LaMack, X.M. Li and M.H. Friedman, "Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability," Heart and Circulatory Physiology, 286, H1916-H1922 (2004).
18. Hinderliter, A. and M. Caughey, "Assessing Endothelial Function As a Risk Factor for Cardiovascular Disease," Current Atherosclerosis Reports, 5, 506 - 513 (2003).
19. Hodgson, L. and J.M. Tarbell, "Solute Transport to the Endothelial Intercellular Cleft: The Effect of Wall Shear Stress," Annals of Biomedical Engineering, 30, 936-945 (2002).
20. Holtzman, J.L., Atherosclerosis and oxidant stress: A new Perspective, Springer-Verlag, New York (2008).
21. Ibragimov, A.I., C.J. McNeal, L.R. Ritter and J.R. Walton, "A mathematical model of atherogenesis as an inflammatory response," Mathematical Medicine and Biology, 22, 305-333 (2005).
22. Ibragimov, A.I., C.J. McNeal, L.R. Ritter and J.R. Walton, "A dynamic model of atherogenesis as an inflammatory response," Advances in Dynamical Systems, 14, 185-189 (2007).
23. Ibragimov, A.I., L.R. Ritter and J.R. Walton, "Stability Analysis of a Reaction-Diffusion System Modeling Atherogenesis," SIAM Journal on Applied Mathematics, 70, 2150-2185 (2010).
24. Ku, D.N., "Blood flow in arteries," Annual Review of Fluid Mechanics, 29, 399-434 (1997).
25. LaMack, J.A., H.A. Himburg, X.M. Li and M.H. Friedman, "Interaction of wall shear stress magnitude and gradient in the prediction of arterial macromolecular permeability," Annals of Biomedical Engineering, 33, 457-464 (2005).
26. Michel, C.C. and F.E. Curry, "Microvascular permeability," Physiological Reviews, 79, 703-761 (1999).
27. Ogunrinade, O., G.T. Kameya and G.A. Truskey, "Effect of Fluid Shear Stress on the Permeability of the Arterial Endothelium," Annals of Biomedical Engineering, 30, 430-446 (2002).
28. Ougrinovskaia, A., R.S. Thompson and M.R. Myerscough, "An ODE model of early stages of atherosclerosis: Mechanisms of the inflammatory response," Bulletin of Mathematical Biology, 75, 1534-1561 (2010).
29. Prosi, M., P. Zunino, K. Perktold and A. Quarteroni, "Mathematical and numerical models for transfer of low-density lipoproteins through the arterial walls: a new methodology for the model set up with applications to the study of disturbed lumenal flow," Journal of Biomechanics, 38, 903-917 (2005).
30. Rappitsch, G. and K. Perktold, "Computer simulation of convective diffusion processes in large arteries," Journal of Biomechanics, 29, 207-215 (1996).
31. Ross, R., "Atherosclerosis - An inflammatory disease," New England Journal of Medicine, 340, 115-126 (1999).
32. Stangeby, D.K. and C.R. Ethier, "Computational analysis of coupled blood-wall arterial LDL transport," Journal of Biomechanical Engineering, 124, 1-8 (2002).
33. Stary, H.C., "Natural history and histological classification of atherosclerotic lesions: an update," Arteriosclerosis, Thrombosis and Vascular Biology, 20, 1177-1178 (2000).
34. Sun, N., N. Wood, A. Hughes, S. Thorn and X. Xu, "Fluid-wall modelling of mass transfer in an axisymmetric stenosis: Effects of shear-dependent transport properties," Annals of Biomedical Engineering, 31, 1-10 (2006).
35. Tada, S. and J.M. Tarbell, "Oxygen mass transport in a compliant carotid bifurcation model," Annals of Biomedical Engineering, 34, 1389-1399 (2006).
36. Tarbell, J.M., "Mass transport in arteries and the localization of atherosclerosis," Annual Review of Biomedical Engineering, 5, 79-118 (2003).
37. Yang, N. and K. Vafai, "Modeling of low-density lipoprotein (LDL) transport in the artery-effects of hypertension," International Journal of Heat and Mass Transfer, 49, 850-867 (2006).
38. Yang, N. and K. Vafai, "Low-density lipoprotein (LDL) transport in an artery - A simplified analytical solution," International Journal of Heat and Mass Transfer, 51, 497-505 (2008).
Received: July 7, 2010.
Accepted: February 11, 2011.
Recommended by Subject Editor Pedro Alcantara Pessoa.












