Servicios Personalizados
Articulo
Latin American applied research
versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.42 no.2 Bahía Blanca abr. 2012
Ultrasound assisted transesterification of corn oil with ethanol
F. A. N. Fernandes†, L. C. A. Mazzone†, L. J. B. L. Matos†, S. J. M. Cartaxo† and S. Rodrigues‡
† Universidade Universidade Federal do Ceara, Departamento de Engenharia Quimica, Campus do Pici, Bloco 709, 60455-760 Fortaleza - CE, BRAZIL, Phone: 55-85-33669611, E-mail: fabiano@ufc.br
‡ Universidade Federal do Ceara, Departamento de Tecnologia dos Alimentos, Campus do Pici, Bloco 858, Caixa Postal 12168, 60421-970 Fortaleza - CE, BRAZIL, Phone: 55-85-33669656, E-mail: sueli@ufc.br
Abstract — This paper evaluates the production of fatty acids ethyl esters from corn oil and ethanol. The reaction was carried out applying low-frequency high-intensity ultrasound (25 kHz) under atmospheric pressure and ambient temperature. Response surface methodology (RSM) was used to evaluate the influence of alcohol to oil molar ratio and catalyst concentration (sodium hydroxide) on the yield of corn oil into ethyl esters. Analysis of the operating conditions by RSM showed that the most important operating condition affecting the reaction was the ethanol to oil molar ratio. Results showed low yield of corn oil into ethyl esters. The highest yield observed was of 62.9% after 30 minutes of reaction. The best operating condition was obtained applying an alcohol to oil molar ratio of 4.5 and a catalyst to oil molar ratio of 0.010.
Keywords — Biodiesel; Ethyl Esters; Ultrasound; Corn Oil; Transesterification; Ethanolysis.
I. INTRODUCTION
Methyl and ethyl esters derived from vegetable oil or animal fat, known as biodiesel, have good potential as an alternative to diesel fuel. Cetane number, energy content, and phase changes of biodiesel are similar to those of petroleum-based diesel fuel (Muniyappa et al., 1996; Darnoko and Cheryan, 2000). Biodiesel fuels have some advantages over petroleum based diesel fuels. Biodiesel fuels are biodegradable, non-toxic and produce less particles, smoke and carbon monoxide.
The conventional mechanical process for biodiesel production is generally carried out in batch reactors. Alcohol, vegetable oil and catalyst are fed and subjected to vigorous agitation and heated to achieve temperatures between 50°C and the boiling point of the alcohol. The time required to achieve total consumption of the oil is about 60 minutes. Alcohol in excess is used to speed up the reaction and to shift the equilibrium toward the formation of products. After the reaction period, the non-converted alcohol has to be separated, purified and recycled back to the reactor (Noureddini et al., 1998; Darnoko and Cheryan, 2000; Stavarache et al., 2005; Meher et al., 2006).
Alternative technologies to produce biodiesel have been studied by several researchers. Among the new technologies some may be suitable to large scale production: ultrasound technology, microwave technology (Mazo and Rios, 2010a) and heterogeneous catalyst technology (Mazo and Rios, 2010b).
Ultrasonic irradiation causes cavitation of bubbles near the phase boundary between the alcohol and oil phases. As a result, micro fine bubbles are formed. The asymmetric collapse of the cavitation bubbles disrupts the phase boundary. Impinging of the liquids creates micro jets leading to intensive mixing of the system near the phase boundary. The cavitation may also lead to a localized increase in temperature at the phase boundary enhancing the transesterification reaction. Neither agitation nor heating are required to produce biodiesel by ultrasound application because of the formation of micro jets and localized temperature increase (Stavarache et al., 2005; Stavarache et al., 2006).
The production of biodiesel using ultrasound has been studied by some researchers. Siatis et al. (2006) have studied the methanolysis of cotton, sunflower and sesame oils obtaining yields into biodiesel ranging from 43 to 93%, which were higher than when the the traditional mechanical process was applied. Colucci et al. (2005) and Santos et al. (2009) have studied the methanolysis of soybean obtaining yields from 69 to 100%. Methanolysis of fish oil was also studied by Santos et al. (2010) and the results showed yields up to 98%.
Staravache et al. (2005) have studied the production of biodiesel using several types of alcohols and found that increasing the length of the chain of the alcohol reduced the yield into biodiesel. Transesterification using methanol resulted in yields from 68 to 98%, while using n-propanol the yield reduced to 92% under the best operating condition.
The amount of catalyst used in the process has a great environmental impact. Large amounts of catalyst tend to produce a larger amount of soap (undesired product) and part of the catalyst remains in the biodiesel increasing its pH. After the end of the transesterification reaction biodiesel is separated from the alcohol phase and then it is washed with water to remove excess catalyst, soap and glycerin, generating large amounts of waste water that needs to be treated.
In this work we have studied the use of low-frequency high-intensity ultrasonic waves to promote the transesterification reaction of corn oil and ethyl alcohol. The reaction was carried out in a batch reactor at ambient temperature using sodium hydroxide as catalyst. The study has carried out with low concentrations of catalyst and low excess of alcohol (lower than the conventional batch process) to verify the efficiency of the ultrasonic process under these conditions.
II. METHODS
A. Materials
Commercial edible grade corn oil was obtained from Bunge Alimentos S.A. (Ipojuca, PE, Brazil) with chemical composition consisting of 57.7% linoleic acid, 29.6% oleic acid and 12.5% palmitic acid (weight percentages). Based on the chemical composition of the oil, its molecular weight was assumed to be 832 g/mol. The oil presented an acid value of 0.4 mgKOH/g, an iodine value of 120 g iodine/100 g oil and a saponification value of 190 mg KOH/ g oil. Analytical grade ethanol (98%) and sodium hydroxide (> 96%) were obtained from Synth (Diadema, SP, Brazil).
B. Transesterification Reaction
Ethanol, corn oil and sodium hydroxide were fed into a glass vessel with nominal volume of 250 mL. The reaction mixture was prepared by mixing the amount of ethanol; soybean oil and sodium hydroxide required to prepare 200 mL of the reaction mixture. The mixture was introduced into the reaction vessel that was placed immersed in water inside an ultrasonic bath (Marconi model Unique USC 40 kHz; internal dimensions: 14 x 24 x 9 cm; volume: 2.7 L; power: 100 W). The reaction was carried out under ambient water temperature (28°C) and atmospheric pressure. Low frequency ultrasound (40 kHz) was applied at a 4870 W/m2 intensity. The ultrasound intensity was determined by the calorimetric method described by Löning et al. (2002). Temperature was maintained constant by circulating water through the ultrasonic bath.
The experiments were carried out following a central composite factorial design with 2 factors (oil to alcohol molar ratio and catalyst to oil molar ratio). The operating conditions are shown in Table 1.
Table 1. Experimental planning used to evaluate the effect of ethanol to oil ratio and catalyst in the production of ethyl esters by ultrasound assisted transesterification (reaction time = 30 minutes, temperature = 28°C).
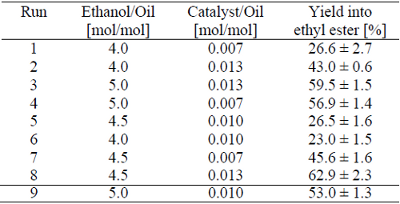
The molar ratio of ethanol and corn oil was set between 4:1 and 5:1, which are below the conventional reaction ratio. The molar ratio of catalyst (sodium hydroxide) to oil was set between 0.007 and 0.013 (used in conventional mechanical biodiesel production). Sodium hydroxide was dissolved into the alcohol before its addition into the reactor. The reaction was carried out during 45 minutes.
After sampling, the reaction mixture was stopped by acidifying the mixture to neutralize the remaining catalyst. The reaction mixture was allowed to stand for phase separation for at least 4 hours. Traces of catalyst and alcohol were washed out with water until the water layer became completely translucent. Finally, the ester layer was dried on anhydrous calcium chloride and analyzed. All experiments were done in triplicate, and the average data are presented.
C. Analysis
The ethyl ester content was assayed by gas chromatograph (Thermos model Ultra) provided with a flame ionization detector. An OV-1 capillary column with 30 m length and 0.25 mm inner diameter and 0.25 μm film thickness was used. The injector and detector temperatures were both set at 250°C. Oven temperature started at 50°C and was increased to 250°C at a rate of 5°C/min and held for 10 min.
III. RESULTS AND DISCUSSION
Experiments were carried out to evaluate the effect of alcohol to oil ratio and catalyst to oil ratio on the transesterification of corn oil with ethanol assisted by ultrasound. The results are presented in Table 1. The yield into ethyl esters showed a maximal yield of 62.9% under the conditions studied herein. The best condition found was using an alcohol to oil ratio of 4.5 mol/mol and a catalyst to oil ratio of 0.013 mol/mol (Table 1). Figure 1 shows the influence of ethanol and catalyst to oil molar ratio on the production of ethyl esters.
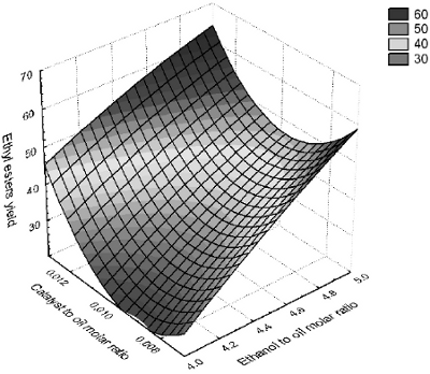
Fig. 1: Yield of corn oil and ethanol into ethyl esters as a function of ethanol to corn oil molar ratio and catalyst to corn oil molar ratio.
The regression equation obtained through surface analysis methodology for the yield of corn oil into fatty acids ethyl esters is given by Eq. (1).
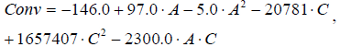 | (1) |
where, A is the ethanol to oil molar ratio and C is the catalyst to oil molar ratio.
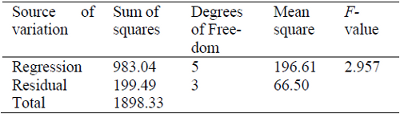
Table 2 presents the analysis of perturbation of factors for the yield of corn oil into ethyl esters. The results show that the main factor influencing the process was the linear factor of the alcohol to oil molar ratio and the quadratic factor of the catalyst to oil molar ratio, which were significant at a 90% level of confidence. Table 3 shows the ANOVA results for the surface analysis methodology. The F-value (2.96) obtained in the ANOVA was higher than the listed F-value (1.80) for 90% level of confidence, indicating that the analysis of perturbation of factors was statistically satisfactory and represents well the data.
Table 2. Analysis of perturbation of yield into ethyl esters caused by factors.
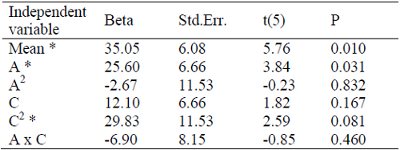
* Significant at 90 % of confidence level.
Table 3. Analysis of variance for yield into ethyl esters.
The dependence of ethyl esters yield on ethanol to oil molar ratio was studied at three different levels (4 - 5 mol/mol) with an increment of 0.5 mol/mol. The yield of ethyl esters was found to be highly dependent on the ethanol to oil molar ratio, which was statistically demonstrated by the low p-value (0.031) (Table 2).
The results clearly demonstrated that the most suitable ethanol to oil molar ratio was 5.0 mol/mol, although optimal yield was also obtained applying a ratio of 4.5 mol/mol and high amount of catalyst. At an ethanol to oil molar ratio of 5.0 mol/mol, the amount of catalyst used in the process is not statistically important and low concentration of catalyst may be applied. This result is important because the use of low catalyst concentration reduces the environmental impact of the waste water produced during the separation process.
The dependence of ethyl esters yield on the amount of catalyst was studied at three different levels of catalyst to oil molar ratio (0.007 - 0.013 mol/mol). The yield into ethyl esters was found to be mildly dependent on this factor. The results demonstrated that the most suitable catalyst to oil molar ratio was 0.013 mol/mol, and that the effect of catalyst concentration is higher at low alcohol to oil molar ratio. The results of the present study did not observe a decrease in ethyl esters yield when the concentration of catalyst was increased, indicating that the conditions applied in this study were below the limit where an increase in the concentration of catalyst decreases the yield into ethyl esters because of formation of soap, which occurs in the presence of high amounts of catalyst (Dorado et al., 2004; Meher et al., 2006).
The yields into ethyl esters obtained in this study were lower than the yields reported in the literature for the transesterification reaction of corn oil with methanol. Staravache et al. (2007) reported yields of 93.1% for methyl esters from corn oil using ultrasound technology. The yield obtained by Staravache et al. (2007) was obtained at a methanol to oil molar ratio of 6:1, temperature of 36°C and a catalyst to oil molar ratio of approximately 0.013 mol/mol. Although the conditions applied by Staravache et al. (2007) differ from the conditions applied in this study, the yield reported by their study is much higher than the reported herein. The yields obtained using methanol and ethanol clearly demonstrates that methanol is the best option for commercial production of fatty acid alkyl esters from corn oil.
The production of ethyl ester from corn oil may become viable if higher alcohol to oil ratios and longer processing times are applied, but at this operating condition the processing cost increases and an economical evaluation of the process is required for commercial use of the process.
IV. CONCLUSIONS
In this work the transesterification of corn oil with ethanol was carried out in a batch reactor subjected to ultrasonic waves. The yield of corn oil into ethyl esters was between 23 and 63% depending on the operating condition applied. The ethyl esters yield obtained from this study were lower than the yield obtained for corn oil methyl esters.
ACKNOWLEDGEMENTS
The authors thank the Brazilian funding institutes CNPq and CAPES for the award of a scholarship and Banco do Nordeste do Brasil for the financial support.
REFERENCES
1. Colucci, J.A., E.E. Borrero and F. Alape, "Biodiesel from an alkaline transesterification reaction of soybean oil using ultrasonic mixing," JAOCS, 82, 525-530 (2005).
2. Darnoko, D. and M. Cheryan, "Kinetics of palm oil transesterification in a batch reactor," JAOCS, 77, 1263-1267 (2000).
3. Dorado, M.P., E. Ballesteros, F.J. Lopez and M. Mittelbach, "Optimization of alkali-catalyzed transesterification of Brassica carinata oil for biodiesel production," En. Fuel, 18, 77-83 (2004).
4. Löning, J.M., C. Horst and U. Hoffmann, "Investigations on the energy conversion in sonochemical processes," Ultrason. Sonochem., 9, 169-179 (2002).
5. Mazo, P.C. and L.A. Rios, "Esterification and transesterification assisted by microwaves of crude palm oil. Homogeneous catalysis," Latin Am. Appl. Res., 40, 337-342 (2010a).
6. Mazo, P.C. and L.A. Rios, "Esterification and transesterification assisted by microwaves of crude palm oil. Heterogeneous catalysis," Latin Am. Appl. Res., 40, 343-350 (2010b).
7. Meher, L.C., D. Vidya Sagar and S.N. Naik, "Technical aspects of biodiesel production by transesterification - A review," Renew. Sust. En. Rev., 10, 248-268 (2006).
8. Muniyappa, P.R., S.C. Brammer and H. Noureddini, "Improved conversion of plant oils and animal fats into biodiesel and co-product," Biores. Tech., 56, 19-24 (1996).
9. Noureddini, H., D. Harkey and V. Medikonduru, "A continuous process for the conversion of vegetable oils into methyl esters of fatty acids," JAOCS, 75, 1775-1783 (1998).
10. Santos, F.F.P., S. Rodrigues and F.A.N. Fernandes, "Optimization of the production of biodiesel from soybean oil by ultrasound assisted methanolysis," Fuel Proc. Tech., 90, 312-316 (2009).
11. Santos, F.F.P., J.Q. Malveira, M.G.A. Cruz and F.A.N. Fernandes, "Production of biodiesel by ultrasound assisted esterification of Oreochromis niloticus oil," Fuel, 89, 275-279 (2010).
12. Siatis, N.G., A.C. Kimbaris, C.S. Pappas, P.A. Tarantilis and M.G. Polissiou, "Improvement of biodiesel production based on the application of ultrasound: monitoring of the procedure by FTIR spectroscopy," JAOCS, 83, 53-57 (2006).
13. Stavarache, C., M. Vinatoru, R. Nishimura and Y. Maeda, "Fatty acids methyl esters from vegetable oil by means of ultrasonic energy," Ultrason. Sonochem., 12, 367-372 (2005).
14. Stavarache, C., M. Vinatoru and Y. Maeda, "Ultrasonic versus silent methylation of vegetable oils," Ultrason. Sonochem., 13, 401-407 (2006).
15. Staravache, C., M. Vinatoru, and Y. Maeda, "Aspects of ultrasonically assisted transesterification of various vegetable oils with methanol," Ultrason. Sonochem., 14, 380-386 (2007).
Received: December 15, 2010.
Accepted: August 25, 2011.
Recommended by Subject Editor Ana Lea Cukierman.












