Servicios Personalizados
Articulo
Latin American applied research
versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.42 no.2 Bahía Blanca abr. 2012
Simulation of eucalyptus kraft black liquor combustion in industrial recovery boilers
M. Cardoso†, G. A. Avelar Costa†, E. D. de Oliveira† and S. P. Ravagnani‡
† Departamento de Engenharia Química - Escola de Engenharia - Universidade Federal de Minas Gerais (UFMG), Rua Espírito Santo 35 - 6° andar Belo Horizonte - MG - Brasil CEP 3016- 030. mcardoso@deq.ufmg.br
‡ Faculdade de Engenharia Química, Universidade de Campinas (UNICAMP), Caixa Postal 6066 - Campinas - SP - Brasil CEP 13083-970. ravag@feq.unicamp.br
Abstract — In this study, the performance of a "kraft" black liquor recovery boiler was analyzed using WinGEMS, a commercial simulator software. The operational variables and design parameters of a pulp industrial unit in southeastern Brazil provides the input data used in setting up the material and energy balance equations in the simulator program. The simulations allowed the prediction of thermal efficiency as a function of solid content in the liquor, in which an increase of 10% in steam generation was obtained when dry solids were increased from 72% to 100%, the latter being a hypothetical operational condition. This study also determined that in spite of making accurate predictions about the temperature profile along the recovery boiler, WinGEMS does not correctly predict the profile of combustion gases when burning eucalyptus black liquor.
Keywords — Black Liquor; Recovery Boilers; Combustion and Simulations.
INTRODUCTION
The aim of this work is to perform an analysis of the impacts of increasing dry solids content of the black liquor burned in a kraft recovery boiler by using a commercial process simulator.
A - RECOVERY BOILER DESCRIPTION
Figure 1 shows the general layout for the kraft recovery boiler studied (Cardoso, 1998). The boiler consists of a furnace, where black liquor combustion takes place, and auxiliary equipment to generate superheated steam that is further used in the plant. Among the auxiliary equipment, economizers, boiler bank, and superheaters are noteworthy.
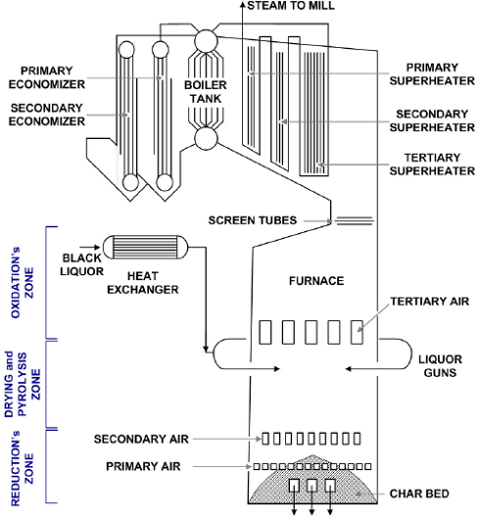
Fig 1: Schematic representation of the recovery boiler analyzed (Cardoso , 1998).
Black liquor is usually pre-heated before being injected into the boiler, turning it less viscous and more easily atomized into the furnace. In the case of the industrial unit analyzed, the concentrated liquor that leaves the evaporator unit passes through a shell and tube heat exchanger reaching temperatures of 110 to 120 °C. This liquor is then sprayed into the furnace, forming droplets that are dispersed and descend counter currently to the hot air and combustion gases causing the drying, devolatilization, and combustion reactions.
Combustion takes place in three different furnace zones (Fig 1), namely, the zones of oxidation, drying and pyrolysis, and reduction of the inorganic compounds with formation of a char bed (Green and Hough, 1992). The oxidation area, located above the liquor injection system, is characterized by the burning of volatile substances produced during the pyrolysis of the liquor. The predominant reactions involve the oxidation of carbon monoxide and sulfur gases, with formation of carbon and sulfur dioxide, respectively. These reactions occur at high temperatures (1400 to 1500 °C) and are extremely fast. The introduction of tertiary air (in excess), in a highly turbulent flow together with the volatile compounds, assure their complete burning. Liquor droplets usually have an initial water content of between 20 and 40% in mass and they generally dry in the region closer to the liquor guns. The size of the droplets, which strongly affects their heat and mass transfer properties, has to be well controlled to guarantee the complete drying of the liquor, otherwise, the char bed will get in contact with water in excess, resulting in its blackout or even explosion. The pyrolysis reactions that take place in the furnace represent the thermal degradation of the solids contained in the liquor. These reactions occur at temperatures above 200°C (Green and Hough, 1992) producing combustion gases (H2S, CO, H2) and a particulate porous solid material, with average diameter of 12 mm. This material descends to the floor of the boiler forming the char bed. The residual fixed carbon, contained in this material, burns in the top layer of the bed (conversion of the fixed carbon to CO and CO2), supplying the necessary heat for the reduction reactions of the inorganic compounds - the reduction region. The final solids, containing mainly sodium carbonate, sulfate, and sulfide are melted and flow to the smelt dissolving tank through water cooled spouts, located in the base of the boiler. Some of this material, however, is partially dragged by the flue gases to the top of the furnace.
Primary air is introduced at the base of the furnace (Fig 1), where it partially oxidizes the organic compounds, supplying heat for the reduction and melting of the inorganic compounds in the char bed. Secondary air is introduced at 1.5 m above the primary air feed line. This air controls the combustion temperature as well as the height and pyramidal shape of the char bed. Tertiary air is necessary to complete the burning of the liquor in the upper part of the furnace. The addition of air in three different levels provides better temperature control in all combustion areas and assures greater boiler efficiency (Vakkilainen, 2005).
The Boiler`s furnace is 11.5 m long, 12 m wide and 32 m high. The boiler has three superheaters with heat exchange areas of 2.104, 2.550 and 2.640 m2. There are also two economizers with heat exchange surface areas of 13.356 and 13.356. The flow of recycled ashes is about to 7% of dry solids in the liquor.
In the modeling the basis for the heat input is the the higher heating value (HHV). About 13.500 to 15.400 kJ/kg are released during the complete combustion of the liquor. This leads to generation of water and sodium sulfate and carbonate, as well as minor quantities with potassium of these compounds. Besides the usual thermal losses of the boiler, this rate of evolved heat supplies the necessary energy (i) to evaporate the water of the fed liquor; (ii) to reduce the sodium sulfate to sulfide; (iii) to melt the inorganic compounds, and (iv) to produce superheated steam (5.4 to 6.4 kg per 1.0 kg of processed pulp or 3.0 to 3.3 kg per 1.0 1.0 kg of black liquor solids). The steam is used in several stages of the process, generating, for instance, electric power for the operation of the plant.
The transfer of heat from the combustion gases to water and steam is done indirectly by the contact of the gases with the walls of tubes and conduits in the boiler where water and steam flow. Water fed to the boiler is heated up in two economizers before being introduced in the boiler bank. From it, the water drains into tubes located in the walls of the boiler, receiving heat by radiation from the char bed and flames in the furnace, and becomes saturated steam. This steam, after reaching the steam drum in the boiler bank, proceeds to the first superheating stage that is constituted by the primary superheater.
As their main function, the screen tubes aim to protect the secondary superheater from the radiation emanated by the furnace. The steam passes by three superheating stages, being the first and the second stage interposed by desuperheaters. Such equipment is designed to homogenize and to control the temperature of the steam so that it remains in the limits specified in the boiler project. At the outlet of the third superheater, it is obtained superheated steam to be used in the process as mentioned above.
B - RECOVERY BOILER MODELING
Two different approaches to model black liquor combustion in recovery boilers have received special attention. The first is Computational Fluid Dynamics (CFD) which is a computational technology that enables the study of interacting particle and gas flow dynamics heat transfer, and combustion processes. For instance, analysis of air injection tactics and their effects on the flue gas patterns can done with CFD techniques and have been used since 1989 to study the local processes inside the furnace. Since then, advances of boiler performance and design have overlapped with technological advances in computer processing capacity and development of mathematical methods for solving differential equations (Ferreira et al., 2010).
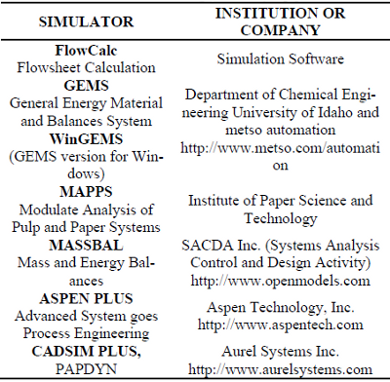
Regarding the second approach, there exist five commercial simulators that, according to Syberg and Wild (1992), are the most appropriate to describe the global black liquor recovery process in paper and pulp industrial plants. These simulators, shown on Tab. 1, are constituted of (i) modular units, representing the several process that occur in the recovery boiler; (ii) a executive program, responsible for the administration of the modular units; and (iii) databases of physicochemical and thermodynamic properties of the components involved in the black liquor combustion process.
Table. 1: Simulators for paper and pulp process.
Shiang and Edwards (1986) developed the models of the modular block, named PCFURN, that describes the combustion of liquor in recovery boilers which is available in the simulator WinGEMS. The advantages of these models are related to the fact of being flexible and capable of representing the combustion of the liquor for diverse boiler configurations. Besides, the block pos sesses detailed modules to describe the operations and phenomena that take place during the liquor combustion stage without requiring long computational time for achieving a solution. The block can also calculate the temperature, pressure, and composition profiles of the combustion gases for the full distance of the boiler (Syberg and Wild, 1992). The accuracy of these predictions are the subject of the present paper. Furthermore, once the block PCFURN is incorporated to WinGEMS simulator, it is possible to simulate the whole black liquor recovery process in an industrial unit. Due to these advantages, WinGEMS simulator has been chosen to describe the combustion stage of eucalyptus black liquor at the studied recovery boiler.
II. METHODS
The methodology used in the simulation (Costa et al., 2007) has been divided in four basic stages: first, the collection of data at the industrial plant; second, the elaboration of the diagram of blocks to describe the boiler; third, the data input to the simulator; fourth, the simulations of the combustion stage.
The information obtained from the plant correspond to (i) project or design data of the furnace and auxiliary equipment (Cardoso, 1998), (ii) boiler operation variables (presented on Table 2), and (iii) data regarding the temperature measurements of the combustion gases, water, and steam at several points of the boiler. The first two series of data represent the entrance or input variables to the simulator, the third set has been compared to the simulation results to test and validate the applied models and methodology.
Table 2: Operational data of the recovery boiler unit analyzed (*).
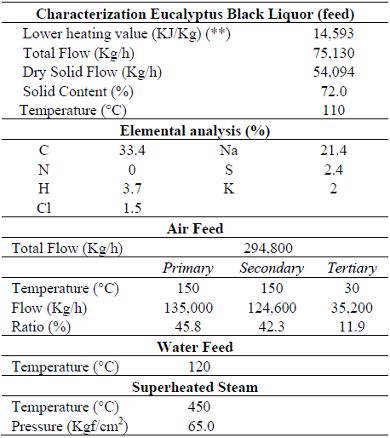
(*) data collected in March, 1996; (**) data from Cardoso et al., 1998 and Cardoso, 1998)
Figure 2 presents a block diagram representation of the recovery boiler. The diagram is composed of interconnected modular units, contained in the WinGEMS simulator, representing the type of recovery boiler used in the industrial plant studied. Table 3 gives an abbreviated description of these units. It should be noted that each unit consists of mathematical equations or empirical correlations that describe each operation taking place during the stage of combustion of the liquor, along regions or sections of the boiler. More details about each of these units can be found in works reported by Uloth et al. (1992), Shiang and Edwards (1986) and Shiang (1986). During the liquor combustion simulation, the executive program retrieves the process variables shared by the modules for entrance or input currents and provides an output of the generated compounds and their concentrations. This executive program also has access to the physicochemical databases supplying, to each module, the necessary information for solving the material and energy balance equations.
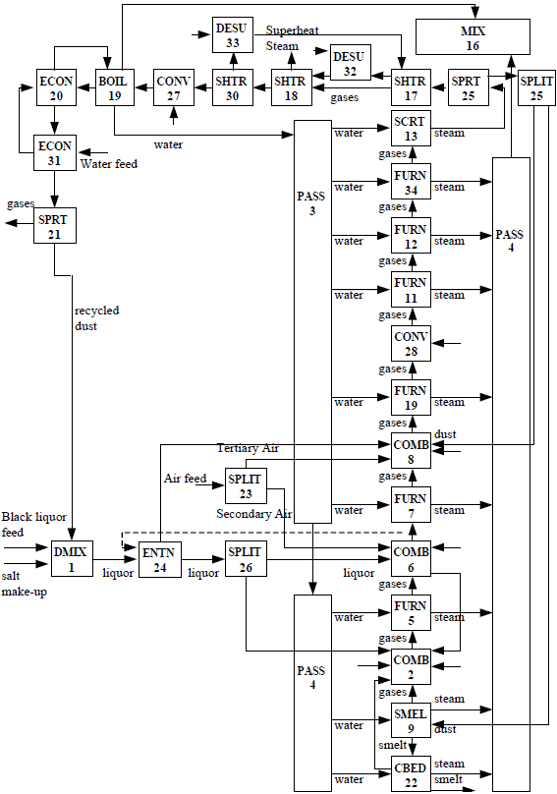
Fig. 2: Block diagram representation of the recovery boiler unit analyzed (Cardoso, 1998)
Table 3: Modular units of PCFURN block in the simulator WinGEMS, Shiang (1986).
The furnace and auxiliary equipment project data are also fed to the simulator, through the diagram of blocks. Such data include: the flow characteristics of gases, air, water, and steam in the several sections of the boiler and inside the ducts and tubes for heat transfer, the thermal exchange area, the dimensions, geometry, and material applied to build the auxiliary equipment, the thermal properties of these materials and, when needed, the thickness of dust deposits incrusted on the external walls of these devices. With this input of the boiler operation data, also via the block diagram, the simulation is allowed to start. After the execution of iterative computations, the simulator provides a final report, containing information regarding the flow, temperature, pressure, solid content, and composition of the several streams presented in Fig. 2.
III. RESULTS AND DISCUSSION
To simulate the combustion of eucalyptus black liquor in the recovery boiler studied, the data displayed on Table 2 was input to WinGEMS with liquor dry solids of 72%.
In Table 4, calculated temperature values for the combustion gases, water, and steam are compared to those measured at strategic points of the boiler. From these figures, it can be observed that the simulation results agree with the boiler temperature range of operation. This shows that the simulator program and applied computation methodology are suitable to simulate the temperature profile along the boiler, despite numerous components and several steps involved in the black liquor combustion simulation.
Table 4: Comparison between simulated and collected data of recovery boiler studied.
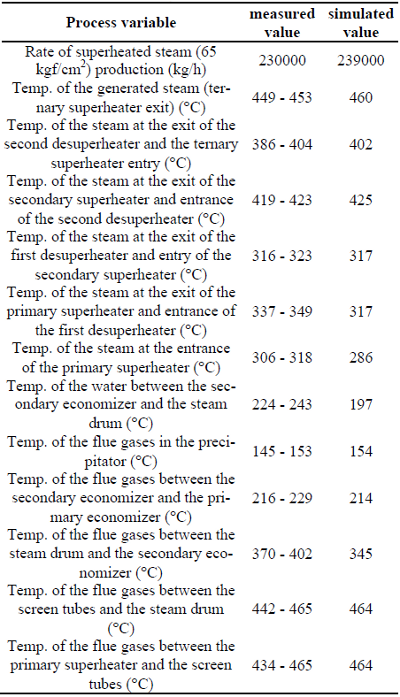
Differently from the temperature profile, when the calculated SO2 emission level is compared to experimental data from the plant, it is observed that the experimental value is much smaller than the simulated result. Based on this fact and also on the models used in SPRT module, it can be asserted that the simulator WinGEMS presents some shortcomings when modeling gas emissions.
Once the simulated temperature profiles of combustion gases and the generated plant steam are close to values obtained in the industrial boiler, it can be concluded that the energy balance and the equations used in its solution are valid to describe the combustion of the liquor. When analyzing the simulated data, it was found that 94.0% of the total energy generated in the boiler comes from the combustion of the organic components. The other 6.0% correspond, mainly, to the energy supplied by the air. As shown in Fig. 3, from the total energy input based on black liquor HHV, about 61.0% (boiler thermal efficiency) is spent to produce steam and 39.0% is lost in several forms (8.5% due to reduction of sulfate to sulfide, 8.0% due to evaporation of water, 6.0% due to sensible heat in dry flue gas, 5.5% due to sensible heat in smelt, 3.5% due to sootblowing steam, uncounted losses 2.0%, and 5.5% due to heat the formation of water from hydrogen in the black liquor).
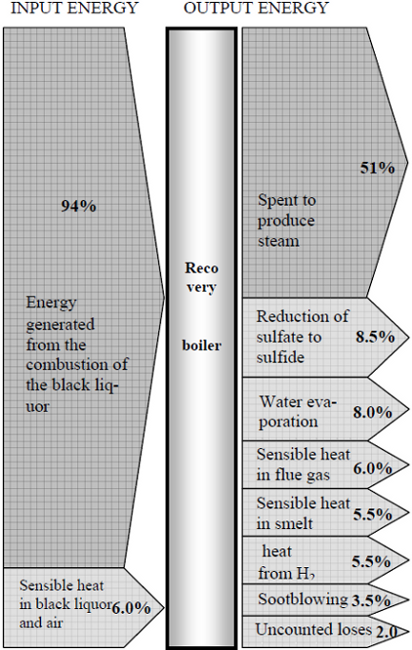
Fig. 3: Energy balance for a kraft black liquor recovery boiler.
Two sources of energy loss deserve special attention. The first corresponds to the heat used in the reduction of sodium sulfate to sulfide (6.5% of the input energy). The second is the amount spent to vaporize the water present in the black liquor (8% of the total). This latter figure can be decreased if the liquor is processed with higher solids content.
To study the effect of increasing solids concentration in the performance of the recovery boiler, the combustion of the liquor has been simulated with increasing levels of solids. As shown on Table 5, the water content in the liquor was altered while the other input parameters were kept constant during these simulations. The results regarding the effects on steam production, boiler thermal efficiency, and operation temperature due to the increase of the solids content in the fed liquor are presented in Figs. 4, 5, 6, and 7 - respectively.
Table. 5: Simulator input variables for the analysis of the effect of increasing liquor solid content in the boiler feed.
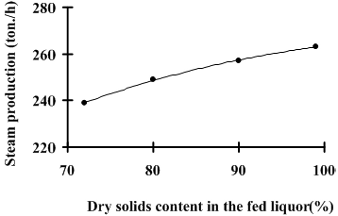
Fig. 4: Simulated superheated steam production rate in function of the liquor dry solids content in the boiler feed.
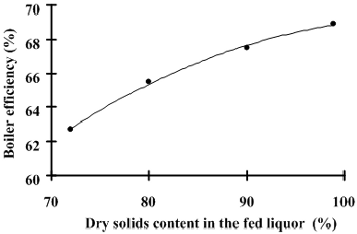
Fig. 5: Simulated value of boiler thermal efficiency in function of the liquor dry solids content in the feed.
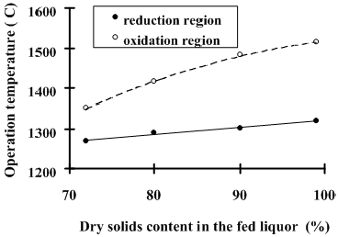
Fig. 6: Simulated temperatures in the oxidation and reduction zones of the recovery boiler in function of the liquor dry solids content in the feed.
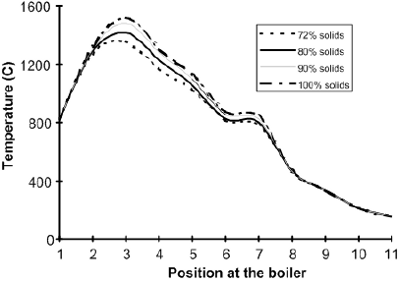
Fig 7a: Simulated profiles of the recovery boiler operation temperature in function of the liquor solids content in the feed (positions identified in Fig 7b).
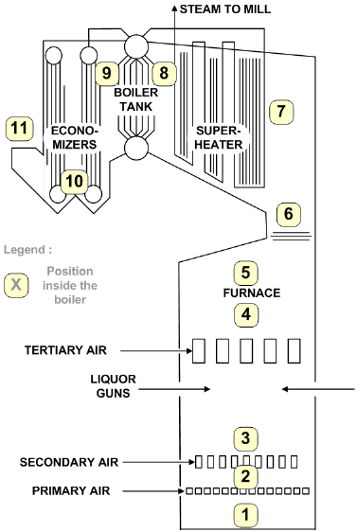
Fig 7b: Positions where operation temperature values (shown in Fig 7a) were calculated using WinGEMS simulator.
It can be observed in Fig. 4 that there is an increase of approximately 10% in the steam production as the concentration of solids in the black liquor fed to the boiler increases from 72% to 99.9% (hypothetical reference operational condition).
Figure 5 indicates an increment in the boiler efficiency as a result of the increase of dry liquor solids content. However, the increase of 10% in the superheated steam production does not represent a net improvement in the global process since the liquor must be concentrated before being injected in the boiler. However, the results presented in Figs. 6 and 7 show that there is a real increment in the boiler operation temperature, mainly in the furnace where the combustion takes place.
According to the data in Fig 6, the increase of liquor dry solids from 72% to 99.9% results in an increment of 50 °C in the operation temperature in the reduction zone and of 160 °C in the oxidation region. This promotes a greater stability of the combustion reactions, avoiding problems related to the explosion or extinction of the char bed. From industrial experience we know that with increasing dry solids, there is an increase in the amount of the combustion products and dust or ashes. Such ashes tend to incorporate sulfur, avoiding its release to the atmosphere.
Studies seeking to develop new technologies aimed at producing a higher level concentration of the black liquor are in process. These technologies represent a major investment of the paper and pulp industry in the next years.
The composition and amount of compounds in the smelt depend on the recovery boiler reduction efficiency and are straightforwardly calculated by solving material balance equations in WinGEMS, which applies a similar methodology as described by Adams et al. (1997). However, mass balance results for flue gas and recycled ashes are limited since WinGEMS does not take into account the reactions of sulfur and sodium compounds in the boiler that strongly influence flue gas and ash compositions (Adams et al., 1997). Such deficiency can be seen in the results shown in Table 6.
Table 6: Simulated and collected data of SO2 e TRS in the flue gas of the recovery boiler studied.
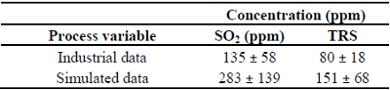
The predicted values by the simulator for SO2 and Total Reduced Sulfur (TRS) in the flue gas of the recovery boiler were nearly 283 and 151 ppm, respecttively. Industrial data indicates much lower values of SO2 and TRS in the gas flue compared to the model predictions. Also, there are large deviations in experimental as well as simulated data (errors usually ranging from 40 to 50%).
Most of the sulfur entering the recovery boiler leaves with the smelt. However, some sulfur is lost from the stack as SO2, TRS gases and Na2SO4 in the dust. The sulfur compounds in the flue gases are formed when hydrogen sulfide and sodium vapors that are released in the furnace react witch each other. These reactions are not considered in the model of the boiler in WinGEMS. The approach adopted in the simulator is based on the minimization of Gibbs energy for twenty seven compounds present in the boiler.
IV. CONCLUSIONS
Increasing the concentration of solids in eucalyptus black liquor before being fed to the recovery boiler represents a real improvement to its recovery process, principally due to the increase of the boiler operation temperature. It has been observed in operating boilers that an increase in solid content improves stability for the liquor combustion reactions as well as an increase in the amount of the products such as ashes. Due to these facts, SO2 emission can be reduced to negligible levels and the recovery boiler can operate at safer and more efficient conditions.
Using the WinGEMS simulator, an increase of 10% has been predicted in the rate of steam production when the dry black liquor solids fed to the boiler is increased from 72% to 99.9% (hypothetical operational condition). Such a gain, however, does not represent a net improvement to the global process since it must be partially or completely used to concentrate the liquor. However, the multiple effect evaporators in the pulp industry work with an economy around 5, which means that a ton of steam evaporates five tons of water from the liquor to be concentrated. Under these circumstances, the increase of solid content is always positive. However, other complications may arise such as increased fouling and viscosity that must be considered when working with more concentrated liquors.
As for the PCFURN module of WinGEMS, it has been found that it is deficient in providing accurate modeling and descriptions of the combustion reactions, mainly those representing the formation of fumes inside the furnace. This leads to combustion gas concentration profiles that do not represent the industrial process analyzed in this work. Studies are in process to improve the modeling in order to evaluate quantitatively the effects of increasing the dry solid content of the black liquor fed to the boiler on the emissions of pollutant gases.
ACKNOWLEDGMENT
This work was sponsored by FAPEMIG and CNPq. Technical support was provided by these industrial plant localized in the southeast of Brazil. The authors are also indebted to Renata Lima of Castro (former chemical engineering undergraduate student and apprentice at the industrial plant) for gathering operational data and Sandro Morais Santos (Chemical Engineer at the industrial plant) for supplying relevant technical information.
REFERENCES
1. Adams, T.N., W.J. Frederick, T.M. Grace, M. Huppa, K. Lisa, A.K. Jones and H. Tran, Kraft Recovery Boilers, Tappi Press, Atlanta (1997).
2. Bennington, C.P.J. and R.J.J. Kerekes, "The effect of temperature on drop size of black liquor sprays," Journal of Pulp and Paper Science, 12, 181-186 (1986).
3. Cardoso, M., Análise da Unidade de Recuperação do Licor Negro de Eucalipto no Processo "Kraft", Avaliando Alternativas de Processamento, Ph.D Thesis, Universidade Estadual de Campinas - UNICAMP, Campinas, Brazil (1998).
4. Cardoso, M., M.L. Passos, F. Carazza and S.M. Santos, "Avaliação Preliminar do Processo de recuperação do licor de Eucalipto para a Otimização", O Papel, LIX, 80 (1998).
5. Costa, G.A.A., E.D. Oliveira, S.W. Park and M. Cardoso, "Overall heat transfer coefficients in a kraft black liquor industrial evaporation unit. Part I - Simulation of multiple effect evaporation system," APPITA Journal, 60, 321-326 (2007).
6. Ferreira, D.J.O., M. Cardoso and S.W. Park, "Gas Flow Analysis in a Kraft Recovery Boiler," Fuel Processing Technology , 91, 789-798 (2010).
7. Green, R.P. and G. Hough, Chemical Recovery in the Alkaline Pulping Process, Alkaline Pulping Committe of the Pulp Manufacture, Tappi Press, 3a ed., Atlanta (1992).
8. Shiang, N.T. and L.L. Edwards, "Kraft Recovery Furnace Modeling and Simulation: Heat Transfer and Gas Flow," AIChE Symposium Series, 81, 81-85 (1985).
9. Shiang, N.T., Mathematical Modeling and Simulation of Recovery Furnaces" Ph.D. Thesis, University of Idaho - Moscow (1986).
10. Syberg, O. and N.W. Wild, Introduction to Process Simulation, Technical Association of Pulp and Paper Industry PRESS, Atlanta, Georgia (1992).
11. Uloth, V., B. Richardson, R. Hogikyan and J. Haynes, "Using a Recovery Boiler Computer Simulation to Evaluate Process Alternatives for Obtaining Incremental Recovery Capacity," Tappi Journal, 75, 137-147 (1992).
12. Vakkilainen, E.K., Kraft recovery boilers: principles and practice, Suomen Soodakattilayhdistys r. y., Helsinki, Finland (2005).
Received: June 1, 2009.
Accepted: June 27, 2011.
Recommended by Subject Editor Orlando Alfano.