Servicios Personalizados
Articulo
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. vol.42 no.3 Bahía Blanca jul. 2012
Asymptotic anaysis for coupled hydrogen, carbon monoxide, methanol and ethanol reduced kinetic mechanisms
A. L. De Bortoli†, ‡ and G. S. L. Andreis‡
† Graduate Program in Applied Mathematics, Federal University of Rio Grande do Sul,
Av. Bento Gonçalves 9500, P. O. Box 15080, Porto Alegre/RS, Brazil
‡ Graduate Program in Chemical Engineering, Federal University of Rio Grande do Sul,
Street Luiz Englert s/n, 90040-040, Porto Alegre/RS, Brazil
dbortoli@mat.ufrgs.br, greice.lorenzzetti@ufrgs.br
Abstract — Based on a mechanism composed by 372 reversible chemical reactions among 56 reactive species for the oxidation of ethanol, we propose a reduction strategy to obtain a sixstep kinetic mechanism for the methanol and a seven-step mechanism for the ethanol. A threestep kinetic mechanism results for the carbon monoxide and two-step for the hydrogen. The reduction strategy consists of four steps: 1) estimate the order of magnitude of the rates of chemical reaction, 2) define the main chain, 3) apply the steady-state and partial equilibrium assumptions and 4) justify the assumptions by asymptotic analysis. The main advantage of the obtained reduced mechanisms is the de-crease of the work needed to solve the system of chemical equations. Such decrease is proportional to the order of the number of elementary reactions present in the complete mechanism.
Keywords — Hydrogen; Carbon Monoxide; Methanol; Ethanol; Reduced Mechanisms.
I. INTRODUCTION
Methanol is commonly used in biodiesel production for its reactivity, and can be employed as one possible replacement for conventional motor fuels (Demirbas, 2007). Methanol has advantages over traditional hydrocarbon fuels derived from mineral oil, because it can be produced from biological sources.
Ethanol can be used as an oxygen additive fuel extender, octane enhancer, or as an alternative fuel to replace reformulated gasoline. Although most ethanol is currently generated by fermentation, recent developments suggest that the ethanol fuel can be derived more efficiently from other types of biomass, thus offering the potential to reduce dependence on fossil-fuel energy resources (Li et al., 2004).
Some mechanisms were obtained and published in the mid-1980s for premixed and nonpremixed flames (Peters and Rogg, 1993). For the oxidation of the hydrogen it is used about 10 chemical species and 20 elementary reactions while for the oxidation of the ethanol it is used about 350 elementary reactions among 50 chemical species (Marinov, 1999).
The computational simulations with detailed mechanisms turn complicated by the existence of highly reactive radicals which induces significant stiffness to the governing equations. Consequently, there exists the need to develop reduced mechanisms of fewer variables and moderate stiffness, while maintaining the accuracy of the detailed mechanism (Lu and Law, 2006).
Kinetic mechanisms for methanol combustion were proposed by Westbrook and Dryer (1980), Dove and Warnatz (1983), Norton and Dryer (1990) and a reduced mechanism based on these works was derived by Paczko et al. (1988). The chemical kinetics of ethanol combustion has been studied by Marinov (1999), Li et al. (2004), Saxena and Williams (2007), Seiser et al. (2007), among others.
Hydrogen is an important intermediate species in the principal path of oxidation of methanol (Seiser et al., 2007). The principal species in the oxidation of methanol (Yalamanchili et al., 2005) and of ethanol are H2O, CO2, CO, H2, O2 and CH2O.
In what follows, we propose a strategy to obtain reduced kinetic mechanisms using the hypotheses of partial equilibrium and of steady-state; we check the reduced mechanisms using an asymptotic analysis and compare some numerical values with data found in the literature.
II. STRATEGY TO OBTAIN REDUCED KINETIC MECHANISMS
The reduction strategy proposed here is to:
-Estimate the order of magnitude for rates of reaction;
-Define the main chain;
-Apply the assumptions of steady-state and of partial equilibrium;
-Justify the assumptions by asymptotic analysis.
The specific velocity k of each elementary reaction is obtained by the relation
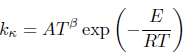 | (1) |
where A is the frequency factor, T the temperature, β the temperature exponent, E the activation energy, and R the gas constant. With these values, it is estimated the magnitude of the rates of reaction and it is defined a main chain for the combustion process.
In a homogeneous system, the assumption of steadystate is valid for those intermediate species that are produced by slow reactions and are consumed by fast reactions, so that their concentrations remain small (Turns, 2000). The partial equilibrium hypothesis is justified when the specific velocities of forward and backward reactions are much larger than all the other reaction specific velocities of the mechanism (Peters, 1988).
A. Reduced Kinetic Mechanisms for Hydrogen and Carbon Monoxide
Wang et al. (1993) presented a reduced mechanism of three-step for wet CO flames that gave reasonable agreement with predictions compared to the mechanism composed by 67 elementary steps among 12 reacting species.
In this work we reproduce the reduced mechanism obtained by Wang et al. (1993) for carbon monoxide, but based on the detailed kinetic mechanism for the oxidation of ethanol presented by Marinov (1999). We note that, like those for the hydrocarbons, the kinetic models for the oxygenated fuels have a logical hierarchy, where the kinetic mechanism of any fuel has, as a subset, the same skeletal mechanism of all fuel smaller molecules (Westbrook et al., 2005).
Consider the reactions 1-20 (hydrogen-oxygen submechanism), 111-114 (HCO consumption) and 126(CO consumption) presented by Marinov (1999), according to the Table 1. They were chosen based on the order of magnitude of the reaction rates and to establish the main chain. Based on the calculation of specific velocities of each elementary reaction, with T = 800 K, we determine the main chain for the carbon monoxide, shown below the dashed line (Fig. 1).
Table 1: Carbon monoxide mechanism rate coefficients units are mol, cm3, s, K and cal/mol).
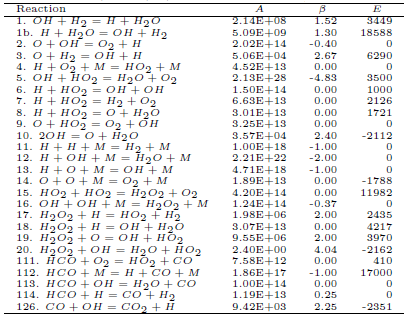
Figure 1: Diagram of the main chain for hydrogen, carbon monoxide, methanol and ethanol.
After applying the hypothesis of partial equilibrium for those reactions with high specific forward and backward velocities, it remains the reactions 1, 3, 11, 12, 13, 14 and 126. Considering the steady-state assumption for the species OH, it results the following three-step mechanism among six species for carbon monoxide
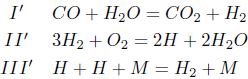
where M is an inert needed to remove the bond energy that is liberated during recombination (Peters, 1992).
In this mechanism, the step I' is the overall CO consumption step, which neither creates nor destroys reaction intermediaries. The step II' represents an overall recombination step, and the step III' an overall radical-production, oxygen-consumption step. The reactions II' and III' constitute the two-step mechanism for the hydrogen.
The reduced mechanism obtained for carbon monoxide can be justified by asymptotic analysis. For the set of elementary reactions presented in the Table 1, the balance equations for the carbon monoxide can be written as
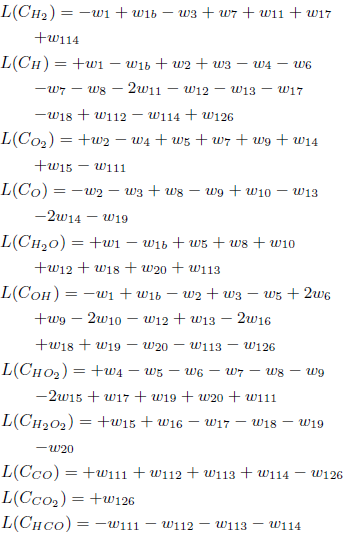
where L(Ci) denotes a linear differential operator applied to the concentration of the species i and wκ represents the reaction rate of the reaction κ. The plus sign refers to species that appear on the right side of an elementary reaction, while the minus sign refers to species on the left. For example, in the reaction 1. OH + H2 = H + H2O, L(COH )= −w1 and L(CH )=+w1, repeating this procedure for all other species and reactions of the mechanism.
Assuming the steady-state hypothesis for the species O, OH, HO2, H2O2 and HCO, their differential operators L are set equal to zero, which leads to five algebraic equations among the reaction rates wκ: w6 = + w4 − w5 − w7 − w8 − w9 − w15 + w16 − w18 + w111, w12 = −w1 + w1b −2w2 +2w4 −3w5 −2w7 −w8 −2w9 −w10 − 2w14 − 3w15 − w16 + w17 + w19 + 2w111 − w126, w13 = −w2 − w3 + w8 − w9 + w10 − 2w14 − w19, w20 = + w15 + w16 − w17 − w18 − w19 and w113 = −w111 − w112 − w114.
Making the rates wI' , wII' and wIII' equal to wI' = w126, wII' = −w2 + w4 −w5 −w7 −w9 −w14 −w15 + w111 and wIII' = −w1 + w1b −3w2 −w3 + 3w4 −3w5 −2w7 −3w9 + w11 − 3w14 − 3w15 + w17 + 3w111 + w114 − w126, one obtains the following linear combinations
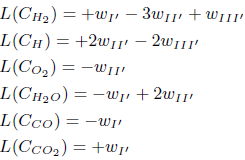
The stoichiometry of these balance equations corresponds to the global mechanism of three-step for the carbon monoxide (I', II', III'), which includes the two-step mechanism for the hydrogen (reactions II' and III' given before).
B. Reduced Kinetic Mechanisms for Methanol and Ethanol
For the methanol, we use the first 129 reversible reactions among 23 species listed by Marinov (1999). Based on the specific velocities of each elementary reaction, with T = 800 K, we determine the main chain for methanol, shown in the Fig. 1.
We apply the hypothesis of partial equilibrium for those reactions with high specific forward and backward velocities; it remains the reactions 1, 3, 7, 11, 12, 13, 14, 43, 109, 111 and 126 (see the tables 1 and 2). The additional application of the steady-state assumption for the species HO2 and OH, results in the following mechanism
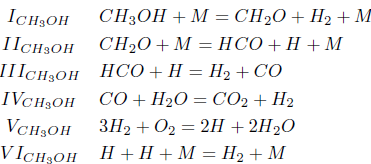
For the asymptotic analysis, consider the reactions with specific velocities less than 5.07 × 109 in addition to the reactions 7 and 111, according to Table 2. Their corresponding balance equations can be written as
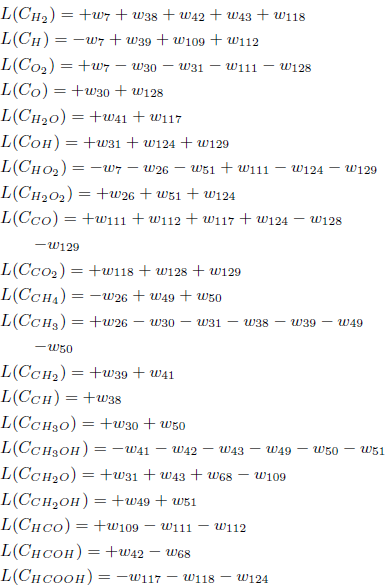
Table 2: Methanol mechanism rate coefficients (units are mol, cm3, s, K and cal/mol). 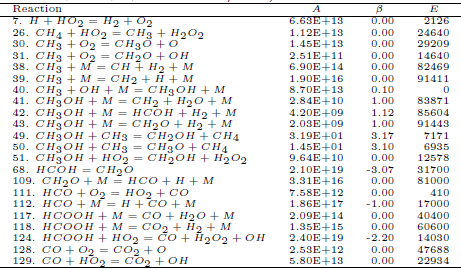
The species O2, O, H2O, OH, HO2, H2O2, CO2, CH2OH, CH3O, CH4, CH3, CH2, CH, HCOOH and HCOH are assumed to be in steady-state and their corresponding L-operators are set equal to zero, what leads to 15 algebraic equations among the reaction rates wκ: w7 = w111 + w112, w26 = −w51, w30 = w38 = w50 = w124 = w128 = 0, w31 = w41 = w118 = w112, w39 = w117 = w129 = −w112, w49 = −w51, w68 = +w42.
Making the rates wI'', wII'' and wIII'' equal to wI''= w42 + w43 + w112, wII'' = w109 and wIII''= w111 + w112, one obtains the following linear combinations
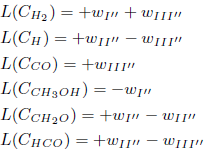
The stoichiometry of these balance equations corresponds to the reactions
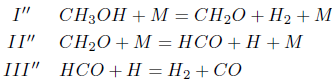
As the carbon monoxide is an important intermediate species in the principal path of oxidation of methanol, we obtain the six-step mechanism for the methanol (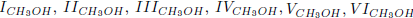 given before), whose reaction rates are given by
given before), whose reaction rates are given by 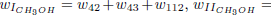
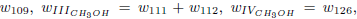
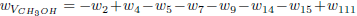 and
and 
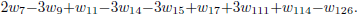 .
.
For the ethanol we consider the 372 elementary reactions among 56 reactive species listed by Marinov (1999). We apply the hypothesis of partial equilibrium for the reactions with high specific forward and backward velocities; it remains the reactions 1, 2, 3, 11, 12, 13, 14, 17, 29, 109, 112, 126, 132, 133, 174, 180, 208 and 224 (see the Tables 1and 3).
The application of the steady-state assumption for the species CH3HCO, C2H2, CH3, O, HCO, H2O2 and OH, results in the following mechanism
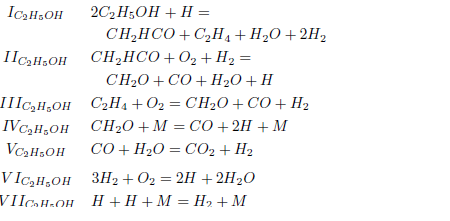
For the asymptotic analysis, consider the reactions with specific velocity less than 4.0 × 10−5 in addition to the reactions 1, 2b, 17, 29, 112, 174, 180 and 224, according to the Table 3. The corresponding balance equations can be written as
Table 3: Ethanol mechanism rate coefficients (units are mol, cm3, s, K and cal/mol).
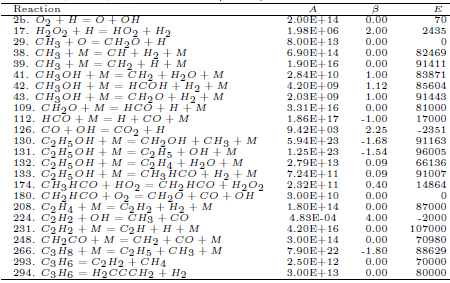
The species O, OH, HO2, H2O2, CH4, CH3, CH2, CH, HCOH, HCO, H2CCCH2, CH2OH, CH3OH, CH3HCO, CH2CO, C2H5, C2H2, C2H, C3H8 and C3H6 are assumed to be in steady-state and their corresponding L-operators are set equal to zero, what leads to 19 algebraic equations among the reaction rates wκ: w1 = +w180 + w208, w2b = −w43 + w208, w17 = +w133, w29 = −w43 + w208, w38 = 0, w39 = +w43, w41 = −w43, w42 = w43 = 0, w112 =+w109, w130 = w131 = 0, w174 =+w133, w224 =+w208, w231 = w248 = w266 = w293 = w294 = 0.
Making the rates wI''', wII''', wIII'''; and wIV''' equal to wI''' =0.5(w132 + w133), wII''' =0.5(w132 − w133 + w180), wIII''' =0.5(−w132 + w133 + w208) and wIV''' = w109, one obtains the linear combinations
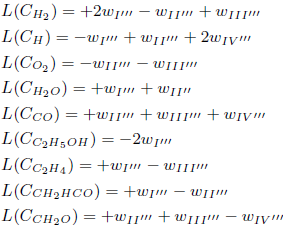
The stoichiometry of these balance equations corresponds to the reactions
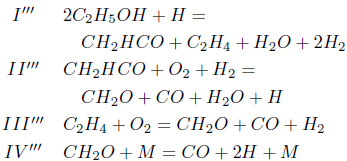
As the carbon monoxide is an important intermediate species in the principal path of oxidation of the ethanol too, we obtain the sevenstep mechanism (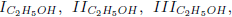 given before), whose reaction rates are given by
given before), whose reaction rates are given by 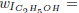
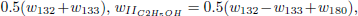
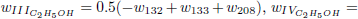
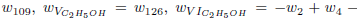
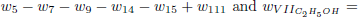
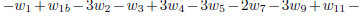
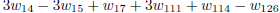 .
.
The coupling of the reactions I', II', III' with I'', II'', III'', and I', II', III' with I''', II''', III''', IV''' is possible because, typically, the mechanisms for hydrocarbon and oxygenated fuels are generated in a hierarchical way, starting with the hydrogen/oxygen system, adding the carbon monoxide subset, followed by the C1 − Cn (Curran, 2009).
III. NUMERICAL RESULTS
Consider a burner whose duct has a cylindrical cross section with De = 1 and a cylindrical tube that injects fuel with d = 0.025, and the burner length is L = 11.
Figure 2 shows the principal combustion products (CO2, H2O) along the mixture fraction space for the methanol and the ethanol. The mixture fraction measures the reactants mixing and is mainly related to the large scale motions of the flow. The maximum value of the mass fraction of the combustion products occurs at proximity of stoichiometric surface (Zst ~ 0.15), where the fuel and the oxidizer mass fractions are both small, since they are consumed at this place.
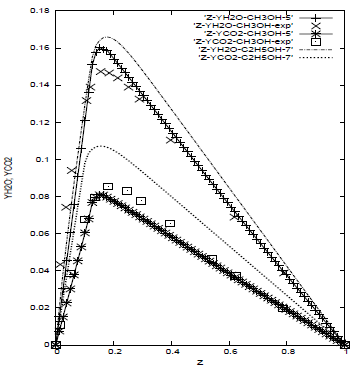
Figure 2: CO2 and H2O mass fractions for the methanol (5-step) compared with the experiment and numerical results for the ethanol (7-step)
The comparison of the principal products (CO2, H2O) for the methanol is done with the experimental values (Müller et al., 1993; Frassoldati et al., 2008), shown in the figure 2. The numerical results agree with the experiment; the mass fraction is slightly overpredicted for the H2O and underpredicted for the CO2 at proximity of the stoichiometric region for the methanol. For the ethanol the tendency is the same.
The main advantage of the strategy is the decrease of the work needed to solve the resultant system of equations. The decrease of time for solving the set of the chemical equations is of one order of magnitude for the hydrogen, since the number of equations decrease in this order. For the methanol and ethanol such reduction in the computational time is of two orders of magnitude.
IV. CONCLUSIONS
The principal contribution of this work is the development of a strategy consisting of 4 steps to obtain reduced kinetic mechanisms for oxygenated fuels, such as the ethanol. We show that there is a coupling between the reduced kinetic mechanisms for the methanol and the ethanol flames, including the mechanisms for the hydrogen and for the carbon monoxide.
From the oxidation mechanism for ethanol presented by Marinov (1999), we obtained reduced mechanisms for the H2, the CO, the methanol and the ethanol. Moreover, the central idea that the kinetic models for common fuels have a hierarchical structure, helps to simplify the analysis.
V. ACKNOWLEDGEMENTS
This research is being developed at UFRGS, Federal University of Rio Grande do Sul. G.S.L. Andreis thanks the financial support from CAPES, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Professor De Bortoli gratefully acknowledges the financial support from CNPq, Conselho Nacional de Desenvolvimento Científico e Tecnológico, under process 303007/2009-5.
REFERENCES
1. Curran, H.J., "Detailed chemical kinetic mechanisms for combustion," Proc. Eur. Combust. Meet. (2009).
2. Demirbas, A., "Progress and recent trends in biofuels," Progr. Energ. Combust. Sci., 33, 1-18 (2007).
3. Dove, J.E. and J. Warnatz, "Calculation of burning velocity and flame structure in methanol -air mixtures," Ber. Bunsen Phys. Chem., 87, 1040-1044 (1983).
4. Frassoldati, A., A. Cuoci, T. Faravelli and E. Ranzi, "Kinetic Modelling of Ethanol Effect on Gasoline Combustion Properties," 31st Meeting on Combustion, 1-6 (2008).
5. Li, J., A. Kazakov and F.L. Dryer, "Experimental and numerical studies of ethanol decomposition reactions," J. Phys. Chem. A, 108, 7671-7680 (2004).
6. Lu, T. and C.K. Law, "Linear time reduction of large kinetic mechanisms with directed relation graph: n-heptane and iso-octane," Combust. Flame, 144, 24-36 (2006).
7. Marinov, N.M., "A detailed chemical kinetic model for high temperature ethanol oxidation," Int. J. Chem. Kinet., 31, 183-220 (1999).
8. Müller, C.M., K. Seshadri and J.Y. Chen, Reduced Kinetic Mechanisms for Applications in Combustion Systems, chapter Reduced kinetic mechanisms for counterflow methanol diffusion flames, Lecture Notes in Physics, Springer-Verlag Berlin, 284-307 (1993).
9. Norton, T.S. and F.L. Dryer, "Toward a comprehensive mechanism for methanol pyrolysis," Int. J. Chem. Kinet., 22, 219-241 (1990).
10. Paczko, G., P.M. Lefdal and N. Peters, "Reduced reaction schemes for methane, methanol and propane flames," 21st Symp. Int. Combust., 21, 739-748 (1988).
11. Peters, N., "Dynamics of Reactive Systems. Part I: Flames, chapter Systematic reduction of flame kinetics: Principles and Details," Progr. Astronaut. Aeronaut., 67-86 (1988).
12. Peters, N.,"Fifteen Lectures on Laminar and Turbulent Combustion," Ercoftac Summer School, Aachen, Germany, Consulted in: 15 July 2008, http://www.itv.rwth-aachen.de /fileadmin/ Lehre-Seminar/ Combustion/ SummerSchool.pdf (1992).
13. Peters, N. and B. Roog, Reduced Kinetic Mechanisms for Applications in Combustion Systems, chapter Preface, Lecture Notes in Physics, Springer-Verlag Berlin Heidelberg, v-vii (1993).
14. Saxena, P. and F.A. Williams, "Numerical and experimental studies of ethanol flames," Proc. Combust. Inst. 31, 1149-1156 (2007).
15. Seiser, R., S. Humer, K. Seshadri and E. Pucher, "Experimental investigation of methanol and ethanol flames in nonuniform flows," Proc. Combust. Inst., 31, 1173-1180 (2007).
16. Turns, S.R., An Introduction to Combustion: Concepts and Applications, McGraw-Hill, Singapore, 2nd ed. (2000).
17.Wang, W., B. Rogg and F.A. Williams, Reduced Kinetic Mechanisms for Applications in Combustion Systems, chapter Reduced kinetic mechanisms for wet CO flames, Lecture Notes in Physics, Springer-Verlag Berlin Heidelberg, 44-57 (1993).
18. Westbrook, C.K. and F.L. Dryer, "Prediction of laminar flame properties of methanol -air mixtures," Combust. Flame, 37, 171-192 (1980).
19. Westbrook, C.K., Y. Mizobuchi, T.J. Poinsot, P.J. Smith and J. Warnatz, "Computational combustion," Proc. Combust. Inst., 30, 125-157 (2005).
20. Yalamanchili, S., W.A. Sirignano, R. Seiser and K. Seshadri, "Reduced methanol kinetic mechanisms for combustion applications," Combust. Flame, 142, 258-265 (2005).
Received: July 17, 2011
Accepted: November 1, 2011.
Recommended by subject editor: Orlando Alfano












