Servicios Personalizados
Articulo
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. vol.42 no.4 Bahía Blanca oct. 2012
Mathematical investigation over the Lewis number effect on the combustion of biomass particles
M. Bidabadi, M. Shokouhmand and M.N. Pour Meibudy
School of Mechanical Engineering, Iran University of Science and Technology, Iran-Tehran
Combustion Laboratory, E-mail: milad.shokouhmand@gmail.com
Abstract — In this paper, the effect of Lewis number on the structure of one-dimensional flame of biomass combustion is analyzed. This effect can be observed by considering the non-unity Lewis number in the governing equations. The fuel particles vaporize first to yield a gaseous fuel of known chemical structure. The analysis is performed in the asymptotic limit, where the value of the characteristic Zeldovich number is too large and the equivalence ratio is larger than unity σ≥u1. The presumed biomass fuel is consisted of four components, moisture, volatile species, fixed carbon and ash. The incineration process of fuel can be described by some chained chemical sub-processes: evaporation of moisture from the fuel particles, volatile release/char formation, burning of the hydrocarbon volatiles in the gaseous space, and the combustion of char and tar components.
The flame structure consists of three different zones which are, a preheat vaporization zone where the rate of the gas-phase chemical reaction is small, a reaction zone that consists of gas and Char combustion where convection and the rate of vaporization of the fuel particles are small and a convection zone where diffusive term in the conservation equation is negligible. The obtained results from the presented model declare that the temperature and burning velocity profiles are extremely affected by the variation of Lewis numbers.
Keywords — Analytical Model; Biomass Combustion; Lewis Number Effect; Burning Velocity
I. INTRODUCTION
Biomass is a clean and renewable source of energy and currently represents nearly 14-15% energy consumption. In fact the necessity of biomass varies significantly across regions of the world. Biomass is becoming a more and usable fuel in the developed countries and because of the diversity of this general source of energy, so many scientists all around the world focus their studies on the different aspects of mathematically, numerically and experimentally modeling of biomass and organic fuels (Chen et al., 1998; William et al., 2001; Backreedy et al., 2002). There are wide ranges of biomass fuels such as wood, short-rotation woody crops and herbaceous species which are studied for at least 20 years. Weng et al. (2006) introduce an extended integral model using char oxidation under different ambient oxygen concentrations to predict the pyrolysis of wood. Also the study of biomass/coal co-firing because of the greenhouse emission is the goal of combustion scientist (Senneca, 2007). SO2 and NOx are alternatively biomass pollutant which can be pyrolysed or gasified producing a liquid fuel or a gas-like fuel such as carbon monoxide. Pyrolysis actually is the thermal destruction processes of organic materials and it is pursued by heating in the absence or shortage of oxygen. Sadhukhan et al. (2008) developed a simple mathematical model to describe the pyrolysis of a single biomass particle. Zheng et al. (2009) studied the pyrolysis characteristics of six representative organic components and their mixtures in a specially designed thermo gravimetric analysis apparatus. Porterio et al. (2006) presented a mathematical model describing the thermal degradation of densified biomass particles. Recently researches mention the explosion ability and pyrolysis of biomass dust particles. Fuel moisture content, fuel volatile content, and in the case of combustion on the fuel bed, air flow rate through the bed are the critical factors affecting the ignition velocity. Yang et al. (2004) present a mathematical model to clarify the effect of primary air flow rate and moisture level in the fuel. Wornat et al. (1996) experimentally studied on the two different type of single particle of biomass char and achieved the burning rates and compare them to the high-volatile bituminous coals. Cho et al. (1995) experimentally studied on burning velocity of multicomponent organic fuel mixture and considered the char, tar and soot formation in the combustion process and plots are illustrated for fuel equivalence ratios between 0.4 to 1.5 at two different ratios and two different temperature. Ryu et al. (2006) experimentally studied over the fuel properties, equivalence ratio and particle size for different particles compositions. Saastamoinen et al. (2001) experimentally and theoretically studied the ignition wave propagation against the air flow in different packed beds of wood fuels and considered the extra factors such as air temperature, bulk density of the fuel bed and particle size. Yang et al. (2003) experimentally studied on the effect of an external heat flux on the pyrolysis of the charring materials and they mathematically modeled the ignition and the combustion model based on Kung's model. They assumed that the chemical reaction between the volatiles and char or between the char and the air are ignorable. Pure fuel chars were compared to blended chars with respect to their performance during combustion is investigated in Kastanaski and Vamvuka (2006) and the kinetic parameters are slightly modified. Cetin et al. (2005) experimentally studied on the influence of Pressure of combustion area on the char structure and gasification kinetics and in their study some results are achieved by the variation of combustion pressure and they analysis the activation energy and also frequency factor in Arrhenius equation. Lu et al. (2010) experimentally and theoretically indicated the influence of particle shape and size on the biomass particle dynamics, including drying, heating and reaction rate.
In the recent studies Bidabadi et al. (2009) present a mathematical model over the effect of propagation rate of dry biomass particle in a fixed bed for analyzing the variation of propagation rate vs. the air flow rate and primary air temperature. The aspects of flame propagation and the structure of combustion zone have been analytically investigated (Bidabadi et al., 2010a) in order to clarify the mechanisms of flame propagation through organic dust cloud. Furthermore Bidabadi et al. (2010b) presented analytical model considering the effect of radiation over the burning velocity in the combustion of biomass particles. In this article the variation effect of Lewis number in a combustion process of biomass fuel particle considering the Char and Tar formation including the heat of vaporization and devolatization of fuel particles is modeled.
II. PHYSICAL MODEL
In this paper, it is assumed the organic particles vaporize first to yield the gaseous fuel of known chemical structure (i.e., methane). In order to analytically investigation of biomass dust particles combustion, the flame structure is composed of three zones; a preheat-vaporization zone, a narrow reaction zone and a post flame zone. The governing equations are energy conservation, gaseous fuel conservation, particle mass fraction conservation and equation of state. Consequently, the final expressions for the flame temperature and burning velocity are obtained by solving these equations in each zone and using the required boundary and matching conditions.
The process particles ignite simultaneously of fuel particles vaporization and Char material is formed and reacts with oxidizer. The picture schematically shows biomass combustion process for dry wood.
During the combustion of biomass particles like wood Six volatile species such as CO, CO2, H2, H2O and one light hydrocarbon and tar are produced and their amounts are based on C, H and O balance with biomass initial analysis and their heating value. Pyrolysis is generally a complex thermochemical process in which lignocelluloses materials within biomass are converted into a gas mixture, and the material which is leaving behind (a carbonaceous residue) known as char. It is assumed that the rate of gas burning velocity and porous materials burning velocities are equal.
Experimental studies result declares that biomass char particles burn over much wider temperature than coal. Char particle temperature range spans the whole range of the theoretical limits (from the lowest rate of burning inert particles to the highest rate of burning diffusion-controlled particles). The rate of flame propagation is depended on the rate of the heat exchanged from the flame and thermodynamical equilibrium of gas phase and solid in the burned zone. In this study the diffusion of pressure gradient and surface reaction are neglected. This model is based on the general combustion process, drying process and pyrolysis. In char combustion process the effect of radiation is ignored. For woods and biomass particles, char material is composed of 58% to 68% of pure carbon and in some cases reaches to 72%.
Cellulose has the lowest char yield, hemicelluloses have the medium and lignin has the highest char yield. Miller and Bellan (1997) describe the char yields as function of temperature for these three compounds. At rang of (~ 520 k - ~ 620 k) the highest rates of char yield for all three compounds are occurred and cellulose has the highest rate of char yield.
Generally the char reactivity is greatly influenced by the nature of the virgin fuel and the reaction conditions during the pyrolysis stage of the thermal conversion. Some physical characteristic factors affect on biomass pyrolysis such as composition of materials, inorganic contents, density, particle size and shape and also some thermodynamical properties such as thermal conductivity, heat capacity and some operating conditions which are temperature, pressure and heating rate. The secondary reaction of tar vapors and conversion time are mainly affected by the variations in the physical properties and the highest influence associated with biomass density and char thermal conductivity (Di Blasi, 1999).
The other parameter which has importantly influence on char combustion for both biomass and coal flame temperature is the air flow rate. Generally flame temperature decreasing with reducing primary air flow rate. At a small air flow rate, a wetter fuel can yield a higher adiabatic flame temperature in the bed than a drier fuel, but the maximum flame front temperature obtainable in the entire air flow range and it decreases with increasing moisture in the fuel. It is obvious that a higher rate of primary air flow or intensification of the moisture level in fuel particles causes shift of the combustion stoichiometry to the fuel-lean side. In this article we assumed that the pressure is constant and the variation of temperature is analyzed by the variation of the characteristic parameter such as Lewis number and equivalence ratio. Yang et al. (2003) are described the specific assumption for establish the mathematical model and they can be modified and used in this study. It assumed that the volatiles produced do not accumulate within the char layer and leave the surface of the fuel at the first zone of combustion immediately. Higher moisture in the fuel results in higher moisture evaporation rate and heightens the char burning, but reduces the devolatilisation rate; totally, burning rate is inversely proportional to the moisture content in the fuel but after all the moisture has been expelled, the surface temperature of the wood becomes higher and the rate of pyrolysis begins to increase and then a reaction zone for pyrolysis is formed. Through the reaction zone propagating inwards, different layers in the fuel decompose simultaneously at different temperatures, because of the temperature field inside the fuel. Study on the propagation of the premixed flames would be useful for describing of the wood ignition and explosion of volatile particles.
III. MATHEMATICAL MODEL
Following the previous models, the structure of premixed flames propagation in combustible system, containing uniformly distributed gaseous fuel, char, and tar and oxidizing gas mixture, is analyzed. In the presented article, the number and radius of the particles are considered to be known as the primary data and it should be noticed that all external forces such as gravitational field on earth are neglected.
The former analysis and experimental attempt at reduced gravity conditions (g/g0 ≤0.01) shows that the local radiative reduction rate is proportional to the value of the density (ns) and the radius of the particle which is transported by this mechanism, is small for low value of ns and also rf /ru<<1, where rfis radius of the fuel particles in combustion reaction zone. Distinction between the quantities value of  u and
u and  g where
g where  g is the effective gas phase stoichiometry at the reaction zone and will be define and modify in the analysis and the illustrated plots. The considerable role of different Lewis numbers and different particle size on the combustion properties of biomass dust particles such as flame temperature and burning velocity is determined in this research and also the variation of effective equivalence ratio as a function of
g is the effective gas phase stoichiometry at the reaction zone and will be define and modify in the analysis and the illustrated plots. The considerable role of different Lewis numbers and different particle size on the combustion properties of biomass dust particles such as flame temperature and burning velocity is determined in this research and also the variation of effective equivalence ratio as a function of  u is analyzed.
u is analyzed.
Combustion process is modelled as a one-step overall reaction type and it can be parametrically shown: 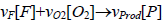 , which F, O2 and P denote the fuel, oxygen and product respectively and the quantities of vF, and vProd are the parametric symbol of their stoichiometric coefficients. The rate of reaction was modelled using the Arrhenius equation, with the parameters quantified using experimental results, k=Bexp(-E/(RT)).
, which F, O2 and P denote the fuel, oxygen and product respectively and the quantities of vF, and vProd are the parametric symbol of their stoichiometric coefficients. The rate of reaction was modelled using the Arrhenius equation, with the parameters quantified using experimental results, k=Bexp(-E/(RT)).
In the preheat-vaporazation zone the rate of reaction between fuel particles and oxidizer is small and its structure is based on balance between the convective, diffusive, and vaporization terms in the conservation equations. The analysis consists on those cases where the overall gas-phase stoichiometry at the reaction zone is fuel-lean. Consequently, excess oxygen would leak from the reaction zone into the convection zone. In the preheat-vaporization zone the rate of chemical reaction between the fuel and oxidizer is presumed to be small. The reaction is assuming as a thin layer which the value of Damköhler number and quantity of Zeldovich number are large enough. The convective and vaporization terms are presumed to be small in comparison with diffusive and also reactive terms; hence these terms are neglected in the governing equations. The burning velocity is determined from analyzing the structure of this zone. in the convection zone since the vaporization process can be continued, chemical reaction between reactant will happen and the diffusive terms in the governing equations are presumed to be small and for simplifying the analysis, this can be ignored in comparison with the convective terms and the represented terms are the gas-phase chemical reaction and vaporization of the volatile fuel particles. Cetin et al. (2005) in their work express that, The char conversion in combustion-based processes fundamentally include the oxidation of char, in gasification, and the char materials can react with a gasifying agent other than oxygen, such as steam or CO2 or some other oxidizers.
The governing equations are written as follow:
Mass Conservation Equation:
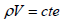 | (1) |
Energy conservation equation:
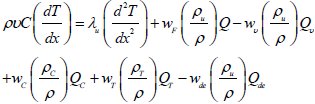 | (2) |
where ρ, v, wF, wc, wt and wυ are the density of the mixture, the flow velocity, the reaction rate, the reaction rate of Char, the reaction rate of Tar and the rate of devolitilization, respectively, and also YF and Yc are the mass fraction of fuel and mass fraction of char, respectively. Also, in this equation λ is the thermal conduction coefficient of both particles and gaseous fuel, and it is proportional to T. Q is the heat released per unit mass of the particles fuel burned in the reaction, Qv is the heat associated with vaporizing unit mass of fuel and C is the heat capacity of Gaseous and fuel particles combination.
Gaseous fuel conservation equation:
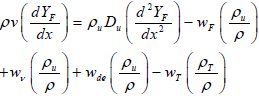 | (3) |
where Y is the mass fraction of particles and D is the mass diffusion coefficient and it is proportional to T2. In Eqs. (2) and (3), the third term is reaction term. In addition, subscripts c, t and de denote char, tar and devolatization, respectively.
Equation of state:
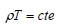 | (4) |
Particle mass fraction conservation equation:
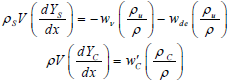 | (5) |
where ρC, ρS and w'c are respectively the density of char, fuel particles and net rate of char generation which are considered to be constant. The heat capacity (C) is a combination of heat capacity of particles (Cs) and gas (CP) and it can be evaluated from this expression:
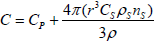 | (6) |
where r is the radius of particles and wv can be described by:
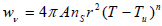 | (7) |
where A is the Characteristic parameter of vaporization rate of fuel particles and n is presumed to be constant. The unit of wv is mass of gaseous fuel vaporized per unit volume per second. Since the rate of vaporization increases with increasing values of T it can be expected to be dominant near the reaction zone, and if
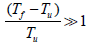
The vaporizing rate can be modified to (Tf-Tu)n.
If the value of ns is presumed to be small, it is logical to assuming the possibility of the volatile fuel particles burning with an envelope surrounding of the diffusion flame for each distributed particles in thin reaction zone and also the analysis developed that the value of nu is large enough such that standoff distance of the envelope flame surrounding each particle is much larger than the obvious characteristic separation distance between the particles which is approximately equal to ns(-1/3).
Williams (1985) express that the flame standoff distance is larger than the separation distance between the liquid droplets if the overall equivalence ratio of the initial combustible mixture is larger than 0.7 and deflagration of spray results is somehow valid for the volatile particles combustion. So this analysis is only valid for fu>0.7 .
The ratio of the devolatalization degree of fuel particles can be defined b1=tr/tv where tr is the transit time of the fuel particles in the preheat-vaporization zone, and tv is the characteristic time for the fuel particle to vaporize completely. In this analysis b1<1 and it is mean that the volatile fuel particle vaporizing process do not complete at the entering of the reaction zone.
Non-dimensional parameters:
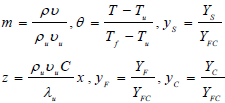 | (8) |
These expressions are introduced into the governing equations, where Tf is the adiabatic flame temperature attained in the reaction zone and the quantity YFC is defined by following expression:
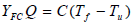 | (9) |
The quantity vu is the characteristic burning velocity of the flame propagation in a combustible mixture of fuel particles in air, calculated by neglecting the heat of vaporization of particles. The particle velocity is presumed to be equal to the gas velocity and the ratio ns/r assumed to be constant. Also some parameters such as ωf, ωde, ωc, γ, q are defined as:
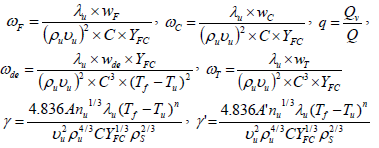 | (10) |
The parameters γ,γ' are presumed to be O(1), and the dimensionless form of the boundary conditions for these equations are:
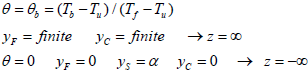 | (11) |
where vu (Characteristic burning velocity of the flame propagation neglecting the particles heat of vaporization) and a is defined by α=YFU/YFC. It is assumed that the quantity of q is negligible which means that the heat released due of reaction is more than the heat absorbed due to vaporization of the particles. In this situation instead of θ, the new parameter (θ 0) can be described where m=1. Hence, the dimensionless governing equations are described as follow:
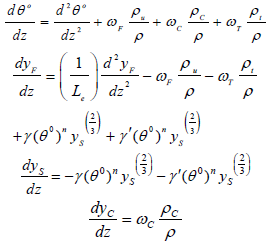 | (12) |
Asymptotic Solution is done in the limit Ze→∞ and γ which is presumed to be of O(1).
IV. ASYMPTOTIC SOLUTION
A. Preheat-Vaporization Zone (-∞ <z<0)
In the limit Ze→∞, the reaction term between the gaseous fuel and oxidizer is assumed negligible. So the first expression of Eq. (12) with the boundary conditions can be obtained:
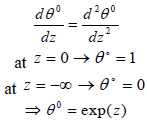 | (13) |
Introducing Eq. (13) into the third expression of Eq. (12) yields:
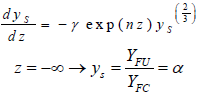 | (14) |
where YFU is the quantity of available fuel particles which is described by
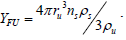
Thus:
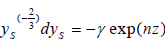 | (15) |
Lewis number can be define as
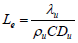
Integrating Eq. (15) using the boundary conditions yields:
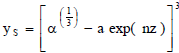 | (16) |
where -[dyf/dz]. Introducing Eqs. (16) and (13) into the second expression of (12) yield:
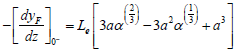 | (17) |
where a=(γ+γ')/(3n).
B. Reaction Zone (z=0)
The reaction zone is divided in three parts. The first one is the combustion of the fuel particles which are converted into the gaseous phase and the second part is the char combustion resultant of the primary fuel particles and the last part is the tar combustion. Convection and vaporization terms are neglected against the diffusion term. Therefore, the governing equations (i.e., Eq. (12)) are rewritten in this zone as:
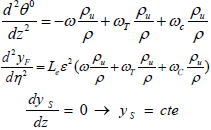 | (18) |
These equations
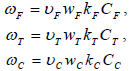
can be used for reaction rate of combustion where CF and wF are respectively, the molar concentration and molecular weight of the fuel and k=Bexp(-Ea/(RTf)). The following equations are used to analyze the structure of the reaction zone:
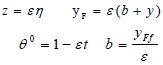 | (19) |
In this equation, ε is presumed to be small and the quantities b and t are presumed to be of order unity. In the limit ε→0, substituting Eq. (19) in the second part of Eq. (12) yields the following expression:
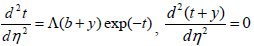 | (20) |
In the above equation, L is a parameter which is presumed to be order of unity and can be expressed as below;
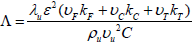 | (21) |
It is assumed that ωCCC=ωFCF=ωTCT.
The results can be obtained using the matching conditions for both preheat-vaporization zone η→-∞ and convection zone η→+∞ with reaction zone condition η→0. In this zone, the convection and vaporization terms are neglected and the temperature is the adiabatic flame temperature which is shown by Tf.
The matching conditions are
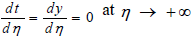
and
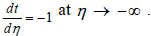
Solving the Eq. (20) results in:
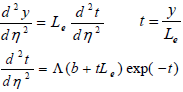 | (22) |
Integration the second part of Eq. (22) using the aforementioned boundary conditions lead to the following expression:
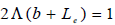 | (23) |
C. Burning Velocity Correlation
Using Eqs. (21) and (23), the burning velocity of fuel particles can be obtained as below:
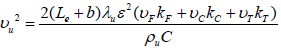 | (24) |
and if two parameters Tf and b are presumed to be known, υu can be calculated. For high value of Tf, it is can be assumed that yFf =0 which is resulted to b=0. Flame temperature (Tf) is evaluated by the jump conditions of preheat-vaporization zone and convection zone. The jump condition is written as:
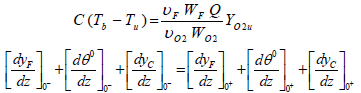 | (25) |
Substituting the Eqs. (13) and (17) for yFf =0, into Eq. (25) results in the following expression for the jump condition:
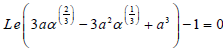 | (26) |
The relation between υu , burning velocity of the flame propagation neglecting the heat of vaporization, and υv, burning velocity of the flame propagation considering the heat of vaporization, can be expressed by the following equation:
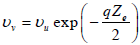 | (27) |
The Zeldovich number which is used in the above equation is described as:
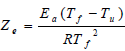 | (28) |
and it is presumed to be a large quantity, the subscript f denotes condition in at the flame zone and the subscript u denotes condition at the ambient reactant stream.
V. RESULTS
In the combustion of the Biomass particles (wood, in this case of study), it is presumed that the fuel particles vaporize first to and the gaseous fuel evolves from the yield the structure similar the methane structure. Submitted results for burning velocity as a function of the primary initial concentration of known organic particles such as methane are available in Coffee et al. (1983).
The laminar burning velocity is defined as the speed relative to the unburned gas, at which a planar, one-dimenstional flame front travels along the direction normal to its surface. It is one of the most importatnt parameters of a combustible mixture.
The chemical kinetic rate parameters are E=25000 Cal/mole and B=5.16×106mol-1s-1. Also the kinetic of vaporization of fuel particles are prescribed by these assumptions: A=3.4×10-4(g)F/[(cm2)Sks], n=1.33.
The constant parameters are presumed as (Smoot and Smith, 1985): λu=3×10-4(cal/cm.s.k), ρu=1.13×10-3(g/cm3), ρS=0.72×10-3(g/cm3), Tu=300 K, q=0.01.
It is shown that for  u≥1 the equivalence ratio based on the fuel available in the fuel particles (
u≥1 the equivalence ratio based on the fuel available in the fuel particles ( u) are formulated by
u) are formulated by  u=17.18YFu/(1- YFu). Also the effective equivalence ratio in the reaction zone (
u=17.18YFu/(1- YFu). Also the effective equivalence ratio in the reaction zone ( g) is calculated by
g) is calculated by  u=17.18 YFC/(1- YFC).
u=17.18 YFC/(1- YFC).
The final adiabatic temperature attained in the convection zone when all the oxygen is consumed, is calculated from the following expression.
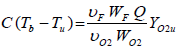 | (29) |
The burning velocity can be predicted by using the values of Β1, Β2 and tr is of the order of λu/(ρuvu2C) and tv is of the order of ρS ru/(3AT) and the characteristic diffusion time
Nine gaseous fuel particles in the direction parallel to the flame front is of the order of ρu C/(4λunu(2/3)). By using the references values for ru, vu, nu, C and T which are set as 20µm, 10cm/s, 3900cm-3, 0.34cal/(gK) and 1200K, respectively, Β1=tr/tv=0.06 and Β2=tr/td=8.0 which is large comparing to the b1 results are obtained for YFU≥0.055 achieving the proper results need to set some parameters as a constant in illustration of the results consequently. In the following illustrations setting the particle sizes as a constant is used to obtaining the effect of Lewis number variation, and for analysis the effect of particle sizes the Lewis number is set as unity. And also for plotting the variation of flame temperature hence to show the approach of temperature change and obtaining the adiabatic flame temperature in this specific case of study the Lewis number is specified at one. The former analytical and experimental studies are showed small particles size has higher burning rates and ignition and also higher front speeds. The mass loss during the ignition propagation period reveals that the amount of char left above the ignition front also increases for larger particles. Large particles are thermally thick having slower rate of devolatilistion than the smaller sizes and more distributed heat transfer to the nearby particles. The particle size also affects the char oxidation period. The temperature gradient at the ignition front also increases with decreasing particle sizes. There are two main reason which it is cause of the devation of illustrated plots comparing to the experimental results of Proust (2009). First thermal radiation effect comes from flame interface into the unburned zone and second the heat recirculation from the surrounding walls of the reaction zone and burned zones to preheat and vaporization zones (Bidabadi et al., 2010a). The flame temperature is calculated via the developed code based on a one-dimensional flame with the Miller-Bowman mechanism and the heat capacity is measured from Eq. (29) and the results for these values as a function of equivalence ratio (rich mixture of methane and air) are shown in Fig. 1. In these calculations, the combustion products are assumed to be CO2, H2O, N2 and CH4. The effects of radiation and heat recirculation are not applied into the governing equations. Generally the Miller-Bowman mechanism is proper code to predicting the burning velocities at lean conditions within experimental error for nearly the entire databases (Cho and Niksa, 1995).
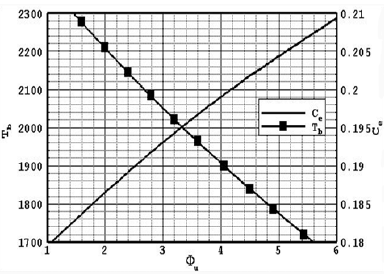
Fig.1. Variation of calculated adiabatic temperature and heat capacity C(cal/gK0) as a function of equivalence ratio
Figure 1 shows the variation of adiabatic temperature and heat capacity as a function of equivalence ratio in the combustion process of biomass cloud particles.
As seen in this figure, decreasing the radius of the particles and shooting up the equivalence ratio is sequent to increasing the burning velocity. It is necessarily needed to note that when the radius of the particles decreases, the number of gaseous and solid particles in the constant control volume increases which is leading to increasing the burning velocity. The variation of υv (burning velocity including the heat of vaporization) and υu (burning velocity neglecting the heat of vaporization) vs. equivalence ratio for different Lewis numbers is depicted in Fig. 2 and the results are compared with the presented data from the numerical and experimental results in the published papers. Zhao and Chen (2011) experimentally studied on the HDMR correlation on investigating the burning velocity which is affected by the percentage of H2 ,O2 for both  >1 and
>1 and  <1. The data portion error less than 5% is much larger for piecewise HDMR correlations. The main discrepancy between the present model and the experimental data is that in this model the structure of flame is divided into three zones and also the effect of devolatization and vaporization for both char and tar materials are considered.
<1. The data portion error less than 5% is much larger for piecewise HDMR correlations. The main discrepancy between the present model and the experimental data is that in this model the structure of flame is divided into three zones and also the effect of devolatization and vaporization for both char and tar materials are considered.
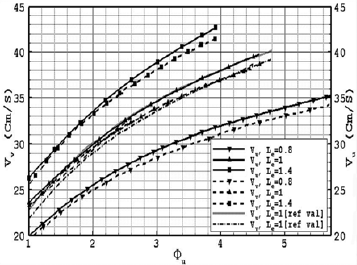
Fig. 2. Variation of burning velocity, including the heat of vaporization, (vv) as a function of equivalence ratio and the variation of burning velocity, neglecting the heat of vaporization vu), as a function of equivalence ratio for different Lewis numbers (ru=20µm).
The illustrated results shows that the increase in Lewis number which is (the ratio of thermal diffusivity to mass diffusivity) associated with acceptable rise in the thermal diffusivity which improves both the vaporization process of volatile fuel particles and the burning velocity of two-phase mixture. In the case in which the heat of vaporization is taken into account, the achieved burning velocity is lower than that while the heat of vaporization is ignored. The concentration of the fuel particles in the vaporization and the reaction zones grows when Lewis number elevated from 0.8 to 1.4 which is probably due to the fact that higher Lewis numbers provide better vaporizing process. The increase in the heat of vaporization associates with the decrease in the burning velocity which physically shows that since heat of vaporization is high; the higher temperature is needed for starting the vaporization process. And it assumed that when the heat of vaporization rise up, mass fraction of biomass dust particles move toward the reaction zone. In this paper the new expression for the vaporization rate of volatile fuel particles has been used in this research in comparison with the available theoretical models and this imply the discrepancy of the results comparing to the experimental results. In Fig. 3, the calculated results for flame temperature and adiabatic temperature are presented for different Lewis numbers, respectively. Increasing the equivalence ratio increases the flame temperature and in the specific equivalence ratio, decreasing the particle radius, associating with the increase in the number of particles, increases the flame temperature. The flame temperature declines by increasing the Lewis number due to the increase in thermal diffusivity per mass diffusivity which denotes that the lower heat is transferred to the combustion flow. Hence, the flame temperature decreases by increasing the Lewis number. As it seen flame temperature lines are limited by the adiabatic flame temperature (Tb)
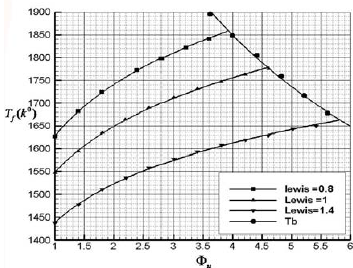
Fig. 3. Variation of flame temperature as a function of equivalence ratio for different Lewis numbers (ru=20µm).
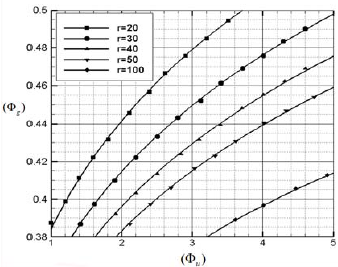
Fig. 4. The effective gas phase equivalence ratio in the reaction zone Φg as a function of Φu for various value of ru(µm).
Figure 5 demonstrates that although Φu is larger than unity, the value of Φg, which is the effective equivalence ratio in the reaction zone, is less than unity. The considerable increasing is seen in the figure probably due to the coupling between the kinetics of vaporization and the kinetics of oxidation. For the achieved value of ru the effective gas phase equivalence ratio in the reaction zone can be assumed as lean for the indicated value of  u which the value of Tb is higher than Tf and for the higher value of fu the presented model because of Tf > Tb is not applicable. And the combustion process can be modeled by fuel-rich combustion.
u which the value of Tb is higher than Tf and for the higher value of fu the presented model because of Tf > Tb is not applicable. And the combustion process can be modeled by fuel-rich combustion.
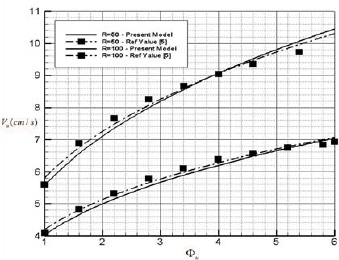
Fig. 5. Variation of burning velocity as a function of Φu for different Radius of particle.
As it seen in Fig. 5 the burning velocity include the heat of vaporization for two different radius of particle ru=50mm and ru=100mm are plotted and compared with the reference value (Bidabadi et al., 2010a) and the main deviation between presented model and referenced value is that in this model the effect of heat of vaporization in charring material have been considered and the devolitilization effect is analyzed mathematically.
In Fig. 6 achieved value of adiabatic flame temperature of this model is compared with the experimental value of Lie et al. (2009) using the chemical reaction analysis to predict and simulate the combustion process of different components of biomass material and illustrate the adiabatic flame temperature of some organic materials which they composed of 55% CH4 , 90% CH4 and 100% CH4 compared with the biomass mixture which is considered in this paper. As it seen in the fuel lean side  <1 the trend of variation for adiabatic flame temperature compared to the 100% CH4 is higher less than 5%.
<1 the trend of variation for adiabatic flame temperature compared to the 100% CH4 is higher less than 5%.
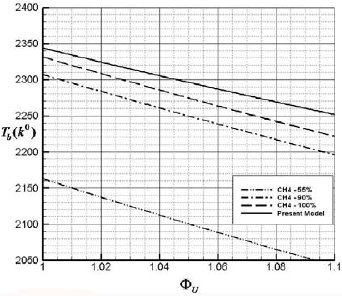
Fig. 6. Adiabatic flame temperature for the indicated equivalence ratio of fuel.
IV. CONCLUSIONS
This article analytically investigates the combustion properties of biomass particles. The initial combustion properties of the biomass particles for instance flame adiabatic temperature and heat capacity of the fuel and air mixture as a function of equivalence ratio are calculated via the standard computer program. The results of this modeling shows that flame is possible to propagating for different values of the equivalence ratio based on the gaseous fuel particles,  u, substantially larger than unity. Also in this paper the variation of burning velocity and the flame temperature via the particles size and thermal properties is shown and illustrated because of the extremely dependence to those parameters. As it shown in the illustrated plot, the burning velocity increases when the equivalence ratio rises. On the other hand, the burning velocity increases when the radius of the fuel particles vary from 100 to 10µm. As it found the contact surface between the oxidizer and the fuel particles has a major effect when the radius of the particle is small. An alternative model can be extended where the effective activation energy of fuel particles vaporization is presumed to be large. It is observed that Lewis number is a determining factor on the combustion properties of biomass particles and increasing the Lewis number coincides with the reduction in the flame temperature. The burning velocity is majorly affected by the variation of the Lewis number as it is illustrated in Fig. 2. It is demonstrated that the burning velocity clearly increases in the indicated values in the modeling. Also the plots imply that the burning velocity increase when the mass fuel particle concentration grows. The higher burning velocity achieved at lower radius of the particle. It means that since the Lewis number rise up, burning velocity augment due to the increase in the thermal diffusivity. A characteristic value temperature Tv could be chosen, such that for particle temperature less than Tv, vaporization effects can be ignored and where the gas temperature is larger than Tv the temperature of the particles is presumed to be constant and has a same value with gas. To describing the condition of steady states flame propagation, non adiabatic consideration concepts are employed. The increase in Tv associated with the decrease in the burning velocity which mathematically and physically express that at higher Tv the higher temperature is needed for starting the vaporization process and the theory declare that since the Tv increase, the mass fraction of the biomass dust particles move towards the reaction zone. It found that excess oxygen would leak from the reaction zone into the convection zone if
u, substantially larger than unity. Also in this paper the variation of burning velocity and the flame temperature via the particles size and thermal properties is shown and illustrated because of the extremely dependence to those parameters. As it shown in the illustrated plot, the burning velocity increases when the equivalence ratio rises. On the other hand, the burning velocity increases when the radius of the fuel particles vary from 100 to 10µm. As it found the contact surface between the oxidizer and the fuel particles has a major effect when the radius of the particle is small. An alternative model can be extended where the effective activation energy of fuel particles vaporization is presumed to be large. It is observed that Lewis number is a determining factor on the combustion properties of biomass particles and increasing the Lewis number coincides with the reduction in the flame temperature. The burning velocity is majorly affected by the variation of the Lewis number as it is illustrated in Fig. 2. It is demonstrated that the burning velocity clearly increases in the indicated values in the modeling. Also the plots imply that the burning velocity increase when the mass fuel particle concentration grows. The higher burning velocity achieved at lower radius of the particle. It means that since the Lewis number rise up, burning velocity augment due to the increase in the thermal diffusivity. A characteristic value temperature Tv could be chosen, such that for particle temperature less than Tv, vaporization effects can be ignored and where the gas temperature is larger than Tv the temperature of the particles is presumed to be constant and has a same value with gas. To describing the condition of steady states flame propagation, non adiabatic consideration concepts are employed. The increase in Tv associated with the decrease in the burning velocity which mathematically and physically express that at higher Tv the higher temperature is needed for starting the vaporization process and the theory declare that since the Tv increase, the mass fraction of the biomass dust particles move towards the reaction zone. It found that excess oxygen would leak from the reaction zone into the convection zone if  u≥1 and
u≥1 and  g≤1. Similarly it is declared that the lower flame temperature and burning velocity are gained for larger size of the particles. Concluding the high temperature vaporization of the fuel particles in convection zone is continued and presumed one-step reaction between vaporized particles and oxygen is expected to be occurred. Gaseous fuel particle mass fraction at both preheats and vaporization zone decrease and increase respectively when the Lewis number increases. Burning separately with a diffusion flame surrounding the particles in convection zone the variation of
g≤1. Similarly it is declared that the lower flame temperature and burning velocity are gained for larger size of the particles. Concluding the high temperature vaporization of the fuel particles in convection zone is continued and presumed one-step reaction between vaporized particles and oxygen is expected to be occurred. Gaseous fuel particle mass fraction at both preheats and vaporization zone decrease and increase respectively when the Lewis number increases. Burning separately with a diffusion flame surrounding the particles in convection zone the variation of  g as a function of
g as a function of  u elucidates that the quantity of
u elucidates that the quantity of  g is less than unity and it does not tend to the stoichiometric condition, while
g is less than unity and it does not tend to the stoichiometric condition, while  u is larger than unity. And it declares that the temperature and burning velocity profiles increase due to increasing the
u is larger than unity. And it declares that the temperature and burning velocity profiles increase due to increasing the  u even for the large values of Β2 cloud particles combustion occurred in the reaction zone. The model declare that the increase in Tv reaches to the level which the amount of the gaseous fuel mass fraction and the onset of the vaporization and highest level of the plot move forward the reaction zone and it expresses the major influence of Lewis number which lead to the flame approaches towards the reaction zone. In the particle cloud combustion including the vaporization of particles which is happened in the convective zone the rate of oxidation of H2 and CO to H2O and CO2 can be ignored.
u even for the large values of Β2 cloud particles combustion occurred in the reaction zone. The model declare that the increase in Tv reaches to the level which the amount of the gaseous fuel mass fraction and the onset of the vaporization and highest level of the plot move forward the reaction zone and it expresses the major influence of Lewis number which lead to the flame approaches towards the reaction zone. In the particle cloud combustion including the vaporization of particles which is happened in the convective zone the rate of oxidation of H2 and CO to H2O and CO2 can be ignored.
NOMENCLATURE
A: Parameter characterizing rate of vaporization of fuel particles
YFC: Parameter characterizing rate of vaporization of fuel particles
a: Defined in Eq. 17.
B: Frequency factor characterizing rate of gas Phase oxidation of the gaseous fuel.
b=yFf/ε: Scaled mass fraction of fuel at the boundary between the reaction zone and the convection zone
C: Heat capacity of mixture
CF: Molar concentration of fuel
CP: Heat capacity of the gas
CS:: Heat capacity of a fuel particle
D: Diffusion coefficient
E: Activation energy characterizing the gas-Phase reaction
k: Constant Rate of the gas-phase reaction
Le: Lewis Number
m: defined in Eq. 8
n: Temperature exponent characterizing rate of vaporization of fuel particles
ns: Local number density of particles (number of particles per unit volume)
nu: Number density of particles in ambient reactant stream (number of particles per unit volume)
Q: Heat release per unit mass of gaseous fuel consumed
Qv: Heat associated with vaporizing unit mass of fuel
Qc: Heat associated with charring unit mass of fuel
QT: Heat associated with Tarring unit mass of fuel
q: Defined in Eq. 10
R: Gas constant
r: Radius of fuel particle
T: Temperature
t: Defined in Eq. 22
v: Velocity
vu: Burning velocity calculated neglecting heat of vaporization of fuel particles
vv: Burning velocity calculated including heat of vaporization of fuel particle
WF: Molecular weight of gaseous fuel
wv: Rate of vaporization of fuel particles
wde: Rate of devolitilization of fuel particles
wc: Rate of charing of fuel particles
wT: Rate of Taring of fuel particles
wF: Reaction rate characterizing consumption of gaseous fuel
Y: Mass fraction
YFC: Defined in Eq. 9
y: Gaseous fuel available in the particles in the ambient reactant stream Defined in Eq. 22
YFu: Gaseous fuel available in the particles in the ambient reactant stream
yF: Defined in Eq. 8
yS: Defined in Eq. 8
ze: Zeldovich number
z: Scaled independent variable, Eq. 11
Greek Symbols
α=YFu/YFC (Eq. 14)
γ,γ': Defined in Eq. 10
ε=1/Ze: Expansion parameter η: Independent variable defined in Eq. 19
θ: Defined in Eq. 11
θ 0: Value of θ calculated neglecting heat of Vaporization of particles
Λ: Defined in Eq. 21
λ: Thermal conductivity
ρ: Density of the reactant mixture
ρs: Density of a fuel particle
v: Stoichiometric coefficient
 u: Equivalence ratio based on fuel available in the particles in the ambient reactant stream
u: Equivalence ratio based on fuel available in the particles in the ambient reactant stream
 s: Effective equivalence ratio in the reaction zone
s: Effective equivalence ratio in the reaction zone
ω: Defined in Eq. 10
Subscripts
b: Adiabatic conditions after completion of chemical reactions
F: Gaseous fuel
f: Conditions at the reaction zone
s: Fuel particles
u: Conditions in the ambient reactant stream
REFERENCES
1. Backreedy, R.I., J.M. Jones, M. Pourkashanian and A. Williams, "Modelling the reaction of oxygen with coal and biomass chars," Proceedings of the Combustion Institute, 29, 415-422 (2002).
2. Bidabadi, M., M. Shokouhmand, J. Fereydooni and A. Rahbari, "Propagation of the reaction front of dry biomass particles in a fixed bed," 22nd International Colloquium on Dynamics of Explosions and Reactive Systems (ICDERS) (2009).
3. Bidabadi, M., M. Azimi and A. Rahbari, "The Effects of Radiation and Particle Size on the Pyrolysis of Biomass Particles," Proceedings of the Institution of Mechanical Engineering, Part C: Journal of Mechanical Engineering Science, 224, 675-682 (2010a).
4. Bidabadi, M., A. Haghiri and A. Rahbari, "The effect of Lewis and Damköhler numbers on the flame propagation through micro-organic dust particles," International Journal of Thermal Sciences, 49, 1146-1456 (2010b).
5. Cetin, E., B. Moghtaderi, R. Gupta and T.F. Wall, "Biomass gasification kinetics: influences o pressure and char structure," Combust. Sci. and Tech., 177, 765-791 (2005).
6. Chen, Y., S. Charpenay, A. Jensen, M.A. Wójtowicz and M.A. Serio, "Modeling of biomass pyrolysis kinetics," Proceeding of the Combustion Institute, 27, 1327-1334 (1998).
7. Cho, S, and S. Niksa, "Elementary reaction model and correlations for burning velocities of multicomponent organic fuel mixtures," Combustion and flame, 101, 411-427 (1995).
8. Cho, S., D. Marlow and S. Niksa, "Burning velocities of multicomponent organic fuel mixtures drived from various coals," Combustion and flame, 101, 399-410 (1995).
9. Coffee, T.P., A.J. Kotlar and M.S. Miller, "The overall reaction concept in premixed, laminar, steady-state flames. I. Stoichiometries," Combustion and Flame, 54, 155-169 (1983)
10. Di Blasi, C.F, F. Buonanno and C. Branca, "Reactivity of some biomass chars in air," Carbon, 37, 1227-1238 (1999).
11. Kastanaki, E., and D. Vamvuka, "A comparative reactivity and kinetic study on the combustion of coalbiomass char blends," Fuel, 85, 1186-1193 (2006).
12. Liu, C., B. Yan, G. Chen and X.S. Bai, "Structure and burning velocity of biomass derived gas flame," Proceeding of European combustion meeting (2009).
13. Lu, H., E. Ip, J. Scott, P. Foster, M. Vickers and L.L. Baxter, "Effects of particle shape and size on devolatilization of biomass particle," Fuel, 89, 1156-1168 (2010).
14. Miller, R.S., and J. Bellan, "A generalized biomass pyrolysis model based on superimposed cellulose, hemicelluloses and lignin kinetics," Combustion science and technology, 126, 97-137 (1997).
15. Porteiro, J., J.L. Míguez, E. Granada and J.C. Moran, "Mathematical modelling of the combustion of a single wood particle," Fuel Processing Technology, 87, 169-175 (2006).
16. Proust, C., "Flame propagation and combustion in some dust-air mixtures," J. Loass Prev. Process Ind., 19, 89-100 (2009).
17. Ryu, C., Y.B. Yang, A. Khor, N.E. Yates, V.N. Sharifi and J. Swithenbank, "Effect of fuel properties on biomass combustion: Part I. Experiments-fuel type, equivalence ratio and particle size," Fuel, 85, 1039-1046 (2006).
18. Saastamoinen, J.J., M. Horttanainen and P. Sarkomaa, "Ignition Wave Propagation and Release of Volatiles in Beds of Wood Particles," Combustion Science and Technology, 165, 41-60 (2001).
19. Sadhukhan, A.K., P. Gupta and R.K. Saha, "Modelling and experimental studies on pyrolysis of biomass particles," Journal of Analytical and Applied Pyrolysis, 81, 183-192 (2008).
20. Smoot, L.D., and P.J. Smith, Coal combustion and gasification, Plenum, New York (1985).
21. Weng, W.G., Y. Hasemi, and W.C. Fan, "Predicting the pyrolysis of wood considering char oxidation under different ambient oxygen concentrations," Combustion and Flame, 145, 723-729 (2006).
22. William, A., M. Pourkashanian and J.M. Jones, "Combustion of pulverized coal and biomass," Progress in Energy and Combustion Science, 27, 587-610 (2001).
23. Williams, F.A., Combustion Theory, 2nd ed., Addison-Wesley, Redwood City , CA (1985).
24. Wornat, M., R. Hurt, K. Davis and N. Yang, "Single-particle combustion of two biomass char," Twenty-Sixth Symposiums (International) on Combustion/the Combustion Institute, 3075-3083 (1996).
25. Yang, L., X. Chen, X. Zhou and W. Fan, W., "The pyrolysis and ignition of charring materials under an external heat flux," Combustion and Flame, 133, 407-413 (2003).
26. Yang, Y.B., V.N. Sharifi and J. Swithenbank, "Effect of air flow rate and fuel moisture on the burning behaviour of biomass and simulated municipal solid wastes in packed beds," Fuel, 83, 1553-1562 (2004).
27. Zhao, Z. and Z. Chen, "HDMR correlations for the laminar burning velocity of premixed CH4 /H2/O2/N2/ mixture," International journal of hydrogen energy, In press (2011).
28. Zheng, J., Y.Q. Jin, Y. Chi, J.M. Wen, X.G. Jiang and M.J. Ni, "Pyrolysis characteristics of organic components of municipal solid waste at high heating rates," Waste Management, 29, 1089-1094 (2009).
Received: August 1, 2010
Accepted: December 11, 2011
Recommended by subject editor: Euduardo Dvorkin.












