Servicios Personalizados
Articulo
Latin American applied research
versión impresa ISSN 0327-0793
Lat. Am. appl. res. vol.42 no.4 Bahía Blanca oct. 2012
Synthesis and characterization of carboxyl and acetal latexes by emulsion polymerization. Application to the production of latex-protein complexes for detecting chagas disease
V.S. García†, V.D.G. González†, J. R. Vega†, I.S. Marcipar‡ and L.M. Gugliotta†
† INTEC (Universidad Nac. del Litoral - CONICET), Guemes 3450, Santa Fe 3000, Argentina. lgug@intec.unl.edu.ar
‡ Lab. de Tec. Inmunológica, Fac. de Bioquímica y Cs. Biológicas, Univ. Nac. del Litoral, Santa Fe 3000, Argentina
Abstract — Monodisperse polymer particles with carboxyl and acetal functionalities were synthesized through a two-step emulsion polymerization process. In the first step, latex particles were synthesized by batch emulsion polymerization of styrene (St); and in the second step, the functional monomers (methacrylic acid or acrolein diethyl acetal) were copolymerized with St onto the previously formed polystyrene particles. The synthesized "core-shell" latexes were used as support for their sensitization (by covalent coupling) with two antigenic recombinant proteins of Trypanosoma cruzi (RP1 and CP1). Polymer latexes and latex-protein complexes were characterized by measuring their colloidal stability, average particle size, shell thickness and protein thickness through conductimetric titration, dynamic light scattering, turbidimetry and scanning electron microscopy.
Keywords— Emulsion Polymerization; Functionalized Latex; Latex-Protein Complex; Recombinant Protein; Chagas Disease.
I. INTRODUCTION
The employment of polymer particles as protein carriers in diagnostic kits dates to the middle of the twentieth century. At that time polystyrene (PS) particles with adsorbed specific antibodies against a particular antigen were used as reagents in the so-called agglutination tests (Bolin et al., 1968; Hipp et al., 1970; Smith and Tsáo, 1973; Heymer et al., 1973).
PS particles were broadly used in the early diagnostic kits, but they exhibited an important deficiency: proteins were attached onto the particles only by physical adsorption. The latex-protein complexes obtained by physical adsorption have a limited applicability because the adsorbed protein could slowly be desorbed during storage or denaturated due to the structural rearrangements that they suffer along the adsorption process (Andrade and Hlady, 1986; 1991; Haynes et al., 1994; Basinka and Slomkowski, 1995; Haynes and Norde, 1995; Han et al., 1996). Also, proteins can be removed from the particle surface by adding an emulsifier to the dispersion medium or by increasing its ionic strength.
Along many years researchers have developed synthetic processes to obtain particles with functional groups suitable for the covalent coupling of proteins, without significantly affecting the colloidal stability of the particles and the biological activity of the proteins. Particles functionalization has motivated many efforts for controlling nature, location, distribution and density of the chemical groups on the particle surface for latex application in diagnostic tests (Gibanel et al., 2001; Slomkowski et al., 2002; Choi et al., 2003; Lambert, 2003, Gonzalez et al., 2008a).
Several heterogeneous polymerization techniques are appropriate for synthesizing latex particles. Emulsion polymerization is the most popular one due to the deep understanding of the involved mechanisms. By controlling the experimental polymerization conditions, it is possible to obtain polymer colloids with uniform particles sizes, and controlled compositions, morphologies, and specific surface characteristics, as required for their use in immunodiagnostic tests. Dispersion polymerization is an alternative when particles larger than those produced by the emulsion process are required.
Functionalized latexes are typically produced by emulsion copolymerization. In the unseeded emulsion process, the main hydrophobic comonomer (e.g., St) is polymerized in the presence of small quantities of a hydrophilic functionalization comonomer. In the seeded (or two-step) process, core-shell morphologies are obtained by polymerizing one or more comonomers in the presence of a uniform latex seed (typically a monodisperse PS latex). In this last case, new polymer particles are not generated along the polymerization, and the final particle diameters are reached by a simple growth of the original seed (Ramos et al., 2003; Sanz Izquierdo et al., 2004; Pichot, 2004; Forcada and Hidalgo-Alvarez, 2005; Gonzalez et al. 2008a).
Functionalized particles employed for immunodiagnostic tests should fulfill requirements related to size, surface charge density and colloidal stability. Uniform particle size and surface charge density are useful for increasing the colloidal stability, which must be carefully controlled either during the latex synthesis (by adequately adjusting the recipe) or by a post-stabilization protocol. Electrostatic stabilization can be reached by using initiators (persulfate salts, azo-derivatives), whose decomposition provides ionic charges that are covalently bound at the interface. In addition, the incorporation of ionically charged comonomers usually produces a significant increase in the surface charge density (Fitch, 1997). Combination of both stabilization mechanisms can be obtained when an ionic comonomer (like acrylic or methacrylic acid) is copolymerized by a seeded or a shot-growth process, leading to the formation of a polyelectrolyte layer. As an alternative, steric stabilization can be attained through macromolecular species (Pichot, 2004).
Several recombinant proteins are suitable for the specific diagnosis of Chagas disease, but unfortunately, no known antigenic protein can be recognized by all the chagasic human sera. To address this drawback, combinations of more than one recombinant protein were used to enhance the assay sensitivity (Umezawa et al., 1999). Also, fusion of DNA sequences encoding selected specific regions of antigenic proteins are a powerful method to synthesize genes encoding chimeric proteins to be used in diagnostic tests.
Our previous works aimed at synthesizing and sensitizing carboxylated latexes for producing latex-antigenic protein complexes to be employed in immunoagglutination tests for detecting Chagas disease. In Gonzalez et al. (2008a), core-shell latexes with external carboxyl groups were synthesized through "seeded" batch or semibatch emulsion copolymerizations of St and methacrylic acid onto monodisperse PS latexes. In Gonzalez et al. (2008b), a single recombinant protein of T. cruzi was coupled onto two carboxylated latexes of similar particle diameters and different charge densities. In Gonzalez et al. (2010), PS and core-shell carboxylated latexes were respectively sensitized by physical adsorption and by covalent coupling, with a homogenate of T. cruzi and with a chimeric recombinant protein of T. cruzi.
In this work, core-shell latexes with external carboxyl or acetal groups were first synthesized through emulsion polymerization of styrene/methacrylic acid or of styrene/acrolein diethyl acetal, respectively, onto a PS seed. After their colloidal characterization, antigenic recombinant proteins of T. cruzi were used to sensitize the latexes, with the ultimate aim of producing an immunoagglutination reagent able to detect the Chagas disease. Sensitization was carried out by using the T. cruzi flagellar repetitive antigen (named RP1) and the chimeric recombinant protein CP1, bearing repetitions of RP1 and the T. cruzi shed acute phase antigen (named RP2), expressed in two different vectors: pET24a and pET32a (Camussone et al., 2009; Belluzo et al., 2011). Finally, the produced latex-protein complexes were characterized through light scattering techniques.
II. MATERIALS AND METHODS
A. Synthesis of Functionalized Latex Particles
The styrene (St) monomer (technical grade, Petrobras Energia S.A., Argentina) was vacuum distilled to eliminate inhibitors and other impurities. In the case of polymerizations yielding carboxyl and acetal surface groups, methacrylic acid (MAA, Merk, purity >99%) and acrolein diethyl acetal (ADEA, Fluka, purity >99%) were the functional monomers, respectively. Potassium persulfate (Mallinckrodt, purity >99%) alone or with sodium methabisulfite (Cicarelli, p.a.) as part of a redox system were used as initiators. Sodium bicarbonate (Cicarelli, p.a.) was used as a buffer. Doubly deionized and distilled water (DDI) was used throughout the work.
A 1-L jacketed glass reactor fitted with a stainless steel stirrer and a thermostatic bath were used. A set of reactor connectors permitted the continuous bubbling of nitrogen, the semibatch addition of reagents, and the withdrawing of samples along the reaction.
Monodisperse particles with carboxyl and acetal functionalities were synthesized by a two steps emulsion polymerization process. In the first step, monodisperse PS particles were synthesized by emulsifier-free emulsion polymerization by using the recipe and conditions given in Table 1. The reaction mixture was stirred at a rate of 250 rpm along 1 h at room temperature, under N2 atmosphere (50 cm3/min). The initiator solution was added after reaching the reaction temperature (T=80°C). After polymerization quenching, the final latex was removed from the reactor. Then, the unreacted St and K2S2O8 were eliminated by serum replacement.
Table 1. PS seed latex: batch recipe and final latex characteristics 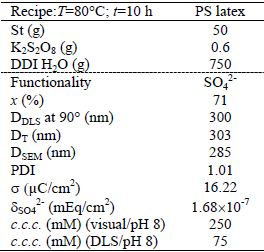
In the second step, the cleaned PS latex was used as a seed for the semibatch and batch copolymerizations carried out for producing carboxyl and acetal latex particles, respectively.
Carboxyl functionalized particles (C2) were synthesized by emulsifier-free semibatch emulsion copolymerization of St and MAA following the method reported by Gonzalez et al. (2008a), with the recipe and conditions given in Table 2. To this effect, the reactor was charged with water, the PS seed, and most of the St monomer; and the mixture was stirred under nitrogen at room temperature for 2 h, to swell the particles with St. Then, the temperature was raised up to 70°C, and a first load of the initiator solution was charged to start the polymerization. After 4 h of reaction, two reagent shots were injected: i) a second load of initiator solution, to increase the final conversion and the final surface density of SO42-; and ii) a mixture of equal masses of St and MAA, to produce the desired functionality while simultaneously avoiding the production of poly(MAA).
Table 2. Carboxylated latex: semibatch recipe and final latex characteristics 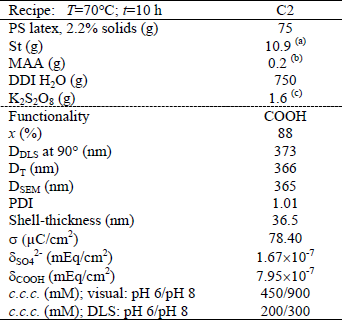
(a) 0.2 g added at 4 h of reaction; (b) added at 4 h of reaction;
(c) 0.8 g added at 4 h of reaction
Two types of acetal-functionalized latex particles (A1 and A2) were prepared by emulsifier-free batch emulsion copolymerization employing the recipe and conditions of Table 3, following the procedure reported by Santos and Forcada (1997) and Sanz Izquierdo et al. (2004). The latex A1 was synthesized at 70°C along 10 h with a St/ADEA ratio of 2:1 (w/w), and K2S2O8 as initiator. The latex A2, was synthesized at 60°C along 6 h with a St/ADEA ratio of 1:2 and the redox couple K2S2O8/Na2S2O5 as the initiator system.
Table 3. Acetal latexes: batch recipes and final latex characteristics 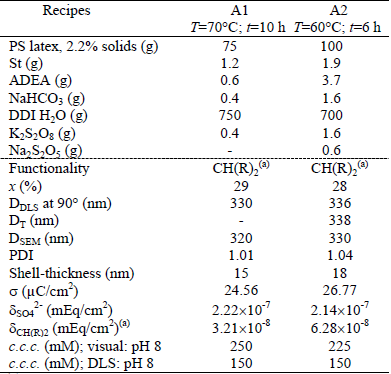
(a) R= OCH2CH3
First, the PS seed was charged into the reactor. Then the aqueous solution of NaHCO3 and the comonomers were incorporated. The reaction mixture was stirred at 250 rpm, under N2 atmosphere (50 cm3/min), for 2 h at room temperature, to swell the particles. Once the reaction temperature was reached, the initiator aqueous solution was charged into the reactor to start the copolymerization.
To purify the functionalized particles, the unreacted comonomers, initiator, and NaHCO3 were eliminated from the final synthesized latexes by serum replacement.
B. Characterization of Polymer Latexes
For all latexes, the following variables were measured: the monomer conversion, the particle size distribution, the shell thickness, the total surface charge density, the functional groups density, and the critical coagulation concentration. Main latex properties are shown in the lower part of Tables 1, 2 and 3.
The final monomer conversion (x) was determined by gravimetry as the ratio of the produced polymer to the total used monomers. The low values of x observed for latexes A1 and A2 may be due to the low solids content of the employed recipes and to the low ADEA reactivity.
The mean particle diameters of the PS seed and the functionalized latexes were determined by Scanning Electron Microscopy (SEM, JEOL-JSM 35C), Dynamic Light Scattering at 90° (DLS, Brookhaven Instruments Inc. with a He-Ne laser at 632.8 nm, and a digital correlator BI-2000 AT), and Turbidimetry (T, UV-vis spectrophotometer, Perkin Elmer Lambda 25). The particle size distribution (PSD) of each latex was determined by SEM on representative samples, and the polydispersity index (PDI) was calculated from the number PSD. In all cases, final latex particles are quasi-monodisperse (1.01 ≤ PDI ≤ 1.04). Notice that the average diameters estimated by DLS (DDLS) and by T (DT) are higher than those obtained by SEM (DSEM) because DLS and T determine hydrodynamic particle diameters, while SEM measures dry particle diameters (Gugliotta and Vega, 2010).
The amount of surface groups was determined by conductimetric titration with an automatic titrator KEM, model At-510 (Garcia, 2010). For the conductivity measurements, the final samples were further diluted with DDI water under magnetic agitation. For the PS and carboxylated latexes, 1 mL of HCl solution (0.1 M) was added to each sample, to produce the complete protonation of the accessible acidic groups (corresponding to the sulfate groups from the persulfate initiator and to the carboxyl groups from the MAA units). For the acetal latexes, the acetal groups were transformed into aldehyde groups by adding HCl 1 M. Then, aldehyde groups were quantified by conductimetric titration, with NaOH, of the released HCl formed in the reaction of these groups with hydroxylamine hydrochloride (Santos and Forcada, 1999). During the titration, the NaOH solution was incorporated in steps, and the conductivity measurements were taken at intervals of 10 s. The total surface charge density (σ) and the density of sulfate groups (δSO42-), carboxyl groups (δCOOH), and acetal groups ( δCH(OCH2CH3)2) are presented in the lower part of Tables 1-3.
The colloidal stability of each latex was evaluated by measuring its critical coagulation concentration (c.c.c.), by direct visualization and by DLS at two different pH (6 and 8), when known concentrations of a KBr solution were added (Garcia, 2010). As expected, the visual c.c.c. resulted higher than those obtained by DLS (see Tables 1-3). The c.c.c. of the acetal and PS latexes were unaffected by pH because the functional groups are deprotonated under both pH. For the carboxylated latex, however, the pH of the medium affected the colloidal stability. At basic pH the carboxyl groups are deprotonated and charged, thus providing an increased stability to the latex particles when compared to acid pH.
C. Production and Purification of Antigenic Recombinant Proteins
The employed recombinant antigens of T. cruzi were the single protein (RP1), and the chimeric protein (CP1) synthesized from the tandem expression of two highly antigenic peptides (RP1 and RP2) expressed in two different vectors: pET32a and pET24a (Camussone et al., 2009; Belluzo et al., 2011). Their main characteristics are presented in Table 4.
Table 4. Characteristics of the employed recombinant proteins of T. cruzi 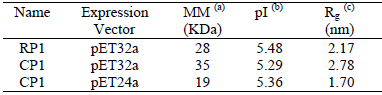
(a) molar mass; (b) isoelectric point (theoretically calculated from the ExPasy Program, http//www.expasy.org/tools/protparam.html); (c) square mean radius using routine g gyrate of the free software Gromacs V. 4.0.7.
E. coli BL21 (DE3) cells bearing the different constructions, pET32a/RP1, pET32a/CP1, and pET24a/CP1, were grown overnight under agitation, in Luria-Bertani (LB) medium, supplemented with 0.1 mg/mL ampicillin (pET32a) or kanamycin (pET24a) at 37°C. Protein expression was induced for 3 h with isopropyl-β-D-thiogalactopyranoside (IPTG). Cells were washed with phosphate-buffered saline (PBS), centrifuged, and resuspended in 50 mM NaH2PO4 (pH 8), 300 mM NaCl, and 20 mM imidazole buffer. Cells were disrupted by sonication (Cleanson), and centrifuged 30 min at 13,000 rpm. The supernatants were applied to Ni-nitrilotriacetic acid column (GE) nickel affinity chromatography. Supernatants were first applied to the columns, and then washed with the same buffer and eluted into different fractions, using the mentioned buffer plus 50, 100, and 250 mM imidazole, consecutively. Then, the purity of the recombinant proteins was analyzed by 15% polyacrylamide gel electrophoresis (PAGE), and stained with Coomassie brilliant blue (Laemmli, 1970).
D. Particles Sensitization by Covalent Coupling
The experimental procedure followed to prepare the latex-protein complexes through covalent coupling consisted of several steps and it was applied to the three core-shell latex particles (C2, A1, and A2). For each latex, a blank without proteins was also prepared.
Carboxyl-functionalized latex particles (C2): recombinant proteins of increasing concentrations (0.3-1.2 mg/mL) were incubated with latex particles (0.10-0.18 m2 of latex surface) in phosphate buffer (pH 5, 2 mM) under stirring along 5 h at room temperature. The activation of carboxyl groups was carried out simultaneously to the covalent coupling to minimize the hydrolysis of the acilurea intermediate. To this effect, the concentration of carbodiimide was selected to be 100-fold greater than the concentration of carboxyl groups, in order to ensure the complete transformation of all the carboxyl groups into acilurea, and to produce a recommended surface density of 10 mg N-N-(3-dimethylamine propyl) N'-ethyl carbodiimide (EDC)/m2 latex. The resulting latex-protein complexes were first isolated from the solution by ultracentrifugation during 30 min at 10,000 rpm, and then they were resuspended in Triton X-100 1% for 24 h, to elute proteins not covalently attached to the particles. Finally, the sensitized particles were resuspended in borate buffer (pH 8) and kept at 4°C. (Gonzalez et al., 2008b).
The total-linked protein (i.e., both physically adsorbed and covalently bound) and the covalently- coupled protein (i.e., the protein that remains on the particle surface after desorption with Triton X-100) were indirectly determined, by measuring the concentrations of dissolved protein through the copper reduction/bicinchoninic acid (BCA) method (Smith et al., 1985).
Acetal-functionalized latex particles (A1 and A2): the first step was the activation of the acetal groups to aldehyde by acidification of the medium with HCl (pH 2), and incubation under continuous mixing for 30 min at room temperature. Then, the sensitization (or protein coupling) step was carried out as follows: the pH was adjusted to 5 by addition of acetate buffer, and the recombinant protein solution was added and gently mixed for 2 h at room temperature. After centrifugation (10,000 rpm for 30 min at 10°C), the particles were resuspended in Triton X-100 for 24 h to elute proteins adsorbed onto the particles. Finally, the sensitized particles were resuspended in the reaction buffer and kept at 4°C. The total-linked and the covalently-bound protein were determined through the BCA method, as described for the carboxylated latex.
The sensitization experiments were made at pH 5 because, under these conditions, the convergence of proteins towards the particle surface is favored by attractive electrostatic interactions. At pH 5 the latex surface has a negative charge, while the recombinant protein has a positive charge according to its isoelectric point (see Table 4). Consequently, the medium conditions must be tightly adjusted to allow the protein to approach the surface of the polymer particles. Otherwise, covalent coupling would be impossible or at least reduced. If there is an electrostatic repulsion between the protein and the polymer surface, it is difficult to produce the covalent bonding of the antigen, because it will not penetrate the particle electrical double-layer. However, if the electrostatic interaction is attractive, covalent coupling will be favored (Gonzalez et al., 2010). Also, the charge of the proteins should not be excessively high to avoid the repulsion between neighboring molecules which would allow a greater amount of protein binds to the surface of the particle. These advantages are obtained when the pH of work is close to the pI of the protein (see Table 4).
In Figs. 1 and 2 the surface densities of total-linked and covalently-coupled protein (GPROT) onto the carboxylated and acetal latexes are represented as a function of the initial protein concentration (C°PROT) at pH 5, for the two considered antigenic proteins, RP1 and CP1. In all cases, the amount of total-linked protein increases with the concentration of added protein.
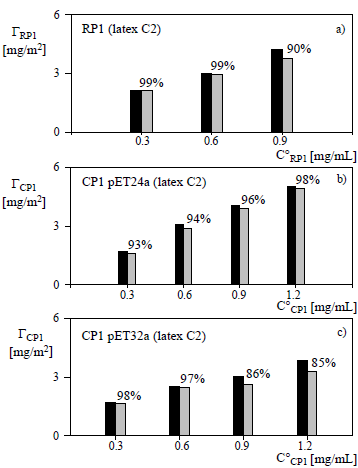
Figure 1: Chemical coupling of RP1 (a), CP1 in pET24a (b), and CP1 in pET32a (c) onto the carboxylated latex at pH 5 vs the initial protein concentration. The surface density of total linked protein (black) is compared with the covalently-bound protein (grey), after desorption with Triton X-100. The percentages on the grey bars indicate the fractions of covalently-coupled protein.
For the carboxylated latex C2, Figs. 1.a-1.c show that the ratio between the total-linked protein and the total added protein decreases when increasing the amount of added protein, and the same tendency is observed with the ratio between the covalently-bound protein and the total added protein. These results could be due to the saturation of the particle surface by the protein. Also, for C°CP1 ≤ 0.6 mg/mL the high fractions of chemically bound RP1, CP1 in pET24a and CP1 in pET32a (> 90%) may render unnecessary the final operation of physical desorption, thus reducing the risk of protein denaturation.
Notice that for CP1 in pET32a, the fraction of covalently bound protein decreases for C°CP1 > 0.6 g/mL
(Fig. 1.c). This may be related to steric hindrance generated between neighboring protein molecules and the shielding of reactive groups attached to the surface due to the larger size of the protein (see Table 4).
For the acetal latexes, Fig. 2 shows that the fractions of covalently-bound proteins results lower than those obtained with the carboxylated latex. Also, the amount of bound protein was unaffected by the increase in the density of surface acetal groups.
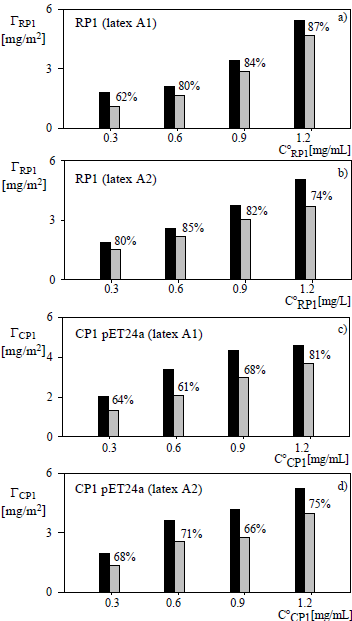
Figure 2: Chemical coupling of RP1 (a, b) and CP1 in pET24a (c, d) onto the acetal latexes A1 (a, c) and A2 (b, d) at pH 5 vs the initial protein concentration (symbols as in Fig. 1).
E. Characterization of Latex-Protein Complexes
Latex-protein complexes were characterized by measuring i) the mean diameters and the protein thicknesses (γ) of the hybrid (latex-protein) particles, by multi-angle dynamic light scattering (MDLS) and by T; and ii) the c.c.c., by DLS at 90°, to evaluate the colloidal stability.
The diameters and the protein thicknesses of the hybrid particles were measured by MDLS at 30°C and the following detection angles: 90°, 100°, 110°, 120° and 130°, to analyze the possible PSD broadening and/or particle agglomeration during the sensitization. The protein thickness was calculated as the difference between the latex-protein particle diameter and the latex particle diameter. The particle concentration was adjusted for each measurement angle in order to obtain a counting rate of about 2'105 counts/s to ensure a regime of simple light scattering. Measure-ment times ranged between 100 and 200 seconds (Gugliotta and Vega, 2010).
Figure 3 shows the protein thicknesses of the latex-protein complexes measured at each detection angle for the C2-CP1 complexes (in pET32a and in pET24a) and for the carboxylated latex C2. The low thicknesses observed at all detection angles indicate the absence of particle agglomeration along the sensitization process. Averaging for all angles, the mean protein thicknesses (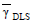 ) were 5.5 and 32.3 nm and the mean latex-protein diameters (
) were 5.5 and 32.3 nm and the mean latex-protein diameters (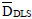 ) were 549 and 493 nm for the C2-CP1 in pET32a and in pET24a vectors, respectively. A higher protein thickness was obtained for the latex-protein particles produced from the CP1 antigen expressed in pET32a. This can be due to the higher molecular weight (and larger hydrodynamic volume) of this protein, as shown in Table 4.
) were 549 and 493 nm for the C2-CP1 in pET32a and in pET24a vectors, respectively. A higher protein thickness was obtained for the latex-protein particles produced from the CP1 antigen expressed in pET32a. This can be due to the higher molecular weight (and larger hydrodynamic volume) of this protein, as shown in Table 4.
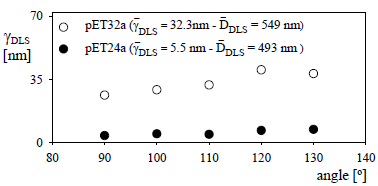
Figure 3: Protein thickness determined by MDLS for the C2-CP1 complex (2.64 mg/m2) expressed in pET24a and in pET32a.
The absorbance spectra of the latex-protein complexes were measured at room temperature, and at the wavelengths range 300-800 nm. To avoid multiple scattering, the complexes were diluted to obtain an absorbance of 1 at 300 nm. Figure 4 shows the results obtained for the C2-CP1 (in pET32a) complex with different amounts of bound protein. In general, when the amount of covalently coupled protein is increased, the absorbance of the latex-protein complex increases, showing a similar trend at different wavelengths. The protein thicknesses of the complexes were measured by DLS at 90° and are reported in the insert of Fig. 4.
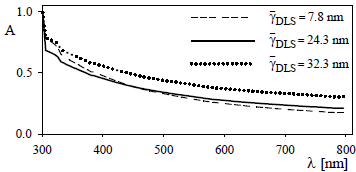
Figure 4: Absorbance spectra for the C2-CP1 complex in pET32a at various concentrations of covalently linked protein (dashed line) 1.65 mg/m2; (continuous line) 2.46 mg/m2, (dotted line) 2.64 mg/m2.
The changes in the absorbance spectrum, A(λ), along the sensitization process are due to variations in both the particle diameter and the particle refractive indexes of the hybrid core-shell latex-protein particles. An increased protein thickness with the amount of initial protein concentration was observed. As a consequence of uncertainties in the refractive indexes, no reliable average diameter can be determined.
Finally, the colloidal stability of the latex-protein complexes obtained from the carboxylated latex C2 and the acetal latex A1 was evaluated on the basis of the c.c.c. measured by DLS at 90° and pH 8, and it was compared to the c.c.c. of the unsensitized latexes (Table 5). The latex-protein complexes exhibited lower stability (lower c.c.c. values) than the un-sensitized latexes. Also, the latex-protein complexes obtained from the carboxylated latex C2 showed greater stability than those obtained from the acetal latex A1.
Table 5. Critical coagulation concentrations determined by DLS at 90° and pH 8. 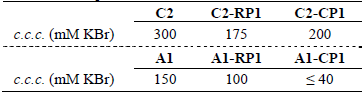
III. CONCLUSIONS
Core-shell latex particles functionalized with carboxyl or acetal groups were synthesized and characterized. The average diameters, the PDI, the c.c.c. values, the surface functional groups and the charge densities showed that the synthesized latexes are uniform in size and present an appropriate stability for their employment in the production of kits for immunoagglutination tests.
The sensitization of the produced functionalized latexes with antigenic recombinant proteins of T. cruzi was carried out with the aim of detecting Chagas disease. Relatively high fractions of chemically-bound protein onto the carboxylated latex were observed with both the single RP1 antigen and the chimeric CP1 (in pET24a) protein for all the initial protein concentrations considered in this work. Thus, the fraction of chemically bound proteins, with respect to the total-linked ones varied between 90% and 99%. For this reason, the physical desorption process after the sensitization could be avoided, thus reducing the risk of protein denaturation. When working with the complex C2-CP1 (in pET32a) the amount of covalently bound protein was slightly lower: between 85% and 98%. This result is probably related to the larger size of the protein which increases the steric hindrance between neighboring macromolecules and produced the occlusion of some surface groups.
Lower fractions of covalently-bound proteins were observed onto the acetal latexes: between 62% and 87%, and it did not vary significantly when increasing the density of surface acetal groups.
From the characterization of latex-protein complexes, it was observed that the complexes showed lower colloidal stability than the unsensitized latexes, with the carboxylated latexes being more appropriate than the acetal ones for immunoaglutination assays. Also, no substantial particle agglomeration occurred during the sensitization process. During such process, the particle diameters are increased, with a simple PSD shift of the latex-protein complexes towards higher values, and with average protein thicknesses between 5 nm (for C2-CP1 in pET24a) and 32 nm (for C2-CP1 in pET32a).
REFERENCES
1. Andrade, J.D. and V. Hlady, "Protein adsorption and materials biocompatibility: a tutorial review and suggested hypotheses," Adv. Polym. Sci., 79, 1-63 (1986).
2. Andrade, J.D. and V. Hlady, "Vroman effects, techniques, and philosophies," J Biomater. Sci. Polymer Edn., 3, 161-172 (1991).
3. Basinka, T. and A. Slomkowski, "Attachment of horseradish peroxidase (HRP) onto the poly(styrene/acrolein) latexes and their derivatives with amino groups on the surface: activity of immobilized enzyme," Coll. and Polym. Sci., 273, 431-438 (1995).
4. Belluzo, M.S., M.E. Ribone, C. Camussone, I. Marcipar and C. Lagier, "Favorable orienting recombinant proteins to develop amperometric biosensors to diagnose Chagas' disease," Anal. Biochem., 408, 86-94 (2011).
5. Bolin, V.S., B. Sue Chase, and J.B. Alsever, "A virus-latex agglutination test for detecting antibodies against isolates associated with viral hepatitis", Am. J. Clin. Path., 49, 635-646 (1968).
6. Camussone, C., V. Gonzalez, M.S. Belluzo, N. Pujato, M.E. Ribone, C. Lagier and I. Marcipar, "Comparation of recombinant Trypanosoma cruzi peptides mixture versus multiepitope chimeric proteins as sensitizing antigens for immunodiagnosis," Clin. Vac. Immunol., 19, 899-905 (2009).
7. Choi, S.W., T.H., Kim, T. Akaike and C.S. Cho, "Surface modification of functional nanoparticles for controlled drug delivery," J. Dispers. Sci. Technol., 24, 475-488 (2003).
8. Fitch, R.M., Chemistry at Interface in Polymer Colloids, A Comprehensive Introduction, Academic Press, New York, 145-172 (1997).
9. Forcada, J. and R. Hidalgo-Álvarez, "Functionalized Polymer Colloids: Synthesis and Colloidal Stability," Curr. Org. Chem., 9, 1067-1084 (2005).
10. Garcia, V.S., Obtención de un Reactivo de Inmunoaglutinación para el Diagnóstico de la Infección por Trypanosoma cruzi, Tesis Doctoral, Univ. Nac. del Litoral, Fac. Ing. Qca., Santa Fe, Argentina (2010).
11. Gibanel, S., V. Heroguez, Y. Gnanou, E. Aramendia, A. Bucsi and J. Forcada, "Monodispersed polystyrene latex particles functionalized by the macromonomer technique. II. Aplication in immunodiagnosis," Polym. Adv. Technol., 12, 494-499 (2001).
12. Gonzalez, V.D.G., L.M. Gugliotta and G.R. Meira, "Latex of immunodiagnosis for detecting the Chagas disease. I. Synthesis of the base carboxylated latex," J. Mater Sci.: Mater Med., 19:777-788 (2008a).
13. Gonzalez, V.D.G., L.M. Gugliotta, C.E. Giacomelli and G.R. Meira, "Latex of immunodiagnosis for detecting the Chagas disease: II. Chemical coupling of antigen Ag36 onto carboxylated latexes," J. Mater Sci.: Mater Med., 19, 789-795 (2008b).
14. Gonzalez, V.D.G., V.S. Garcia, J.R. Vega, I.S. Marcipar, G.R. Meira and L.M. Gugliotta, "Immunodiagnosis of Chagas disease: Synthesis of three latex-protein complexes containing different antigens of Trypanosoma cruzi," Coll. and Surf. B: Biointerf., 77, 12-17 (2010).
15. Gugliotta, L.M. and J.R. Vega, Measurement of Particle Size Distribution of Polymer Latexes, Research Signpost, India (2010).
16. Han, D.K., K.D. Park, G.H. Ryu, U.Y. Kim, B.G. Min and Y.H. Kim, "Plasma protein adsorption to sulfonated poly(ethylene oxide)-grafted polyurethane surface," J. Biomed. Mater. Res., 30, 23-30 (1996).
17. Haynes, Ch.A., E. Sliwinsky and W. Norde, "Structural and electrostatic properties of globular proteins at a polystyrene-water interface," J. Coll. Interf. Sci., 164, 394-409 (1994).
18. Haynes, Ch.A. and W. Norde, "Structures and stabilities of adsorbed proteins," J. Coll. Interf. Sci., 169, 313-328 (1995).
19. Heymer, B., W. Schachenmayr, B. Bultmann, A. Spanel, O. Haferkamp and W. Schmidt, "A latex agglutination test for measuring antibody to streptococcal mucopeptides," J. Immunol., 111, 478-484 (1973).
20. Hipp, S., D.S. Berns, V. Tompkins and H. Buckley, "Latex slide agglutination test for Aspergillus antibodies," Sebouraudia, 8, 237-241 (1970).
21. Laemmli, U.K., "Cleavage of structural proteins during the assembly of the head of bacteriophage T4," Nature, 227, 680-685 (1970).
22. Lambert, G. "Polyalkyleyanoacrylate nanospheres and nanocapsules for the delivery of antisense oligonucleotides," J. Dispers. Sci. Technol., 24, 439-452 (2003).
23. Pichot, C., "Surface functionalized latexes for biotechnological applications," Curr. Op. Coll. Interf. Sci., 9, 213-221 (2004).
24. Ramos, J., A. Martín-Molina, M.P. Sanz-Izquierdo, A. Rus, L. Borque, R. Hidalgo-Álvarez, F. Galisteo-González and J. Forcada, "Amino-funcionalized latex particles obtained by a multistep method: development of a new immunoreagent," J. Pol. Sci. : Part A: Pol. Chem., 41, 2404-2411 (2003).
25. Santos, R.M. and J. Forcada, "Acetal functionalized polymer particles useful for immunoassays," J. Polym. Sci. Part A: Polym Chem., 35, 1605-1610 (1997).
26. Santos, R.M. and J. Forcada, "Acetal functionalized polymer particles useful for immunoassays. II. Surface and colloidal Characterization," J. Polym. Sci. Part A: Polym Chem., 37, 501-511 (1999).
27. Sanz Izquierdo, M.P., A. Martín-Molina, J. Ramos, A. Rus, L. Borque, J. Forcada and F. Galisteo-González, "Amino, chloromethyl and acetal-functionalized latex particles for immunoassays: a comparative study," J. Immunol. Meth., 287, 159-167 (2004).
28. Slomkowski, S., T. Basinska and B. Miksa, "New types of microspheres and microsphere-related materials for medical diagnostic," Polym. Adv. Technol., 13, 906-918 (2002).
29. Smith, R. and Ch. Ts'ao, "Fibrin degradation products in the postoperative period. Evaluation of new latex agglutination method," Am. J. Clinical Pathology, 60, 644-647 (1973).
30. Smith, P., R. Krohn, G. Hermanson, A. Mallia, F. Gartner, M. Frovenzano, E. Fujimoto, N. Goeke, B. Olson and D. Klenk, "Measurement of Protein Using Bicinchoninic Acid," Anal. Biochem., 19, 76-85 (1985).
31. Umezawa, E.S., S.F. Bastos, M.E. Camargo, L.M. Yamauchi, M.R. Santos, A. Gonzalez, B. Zingales, J.M. Levin, O. Souza, R. Rangel-Aldao and J. F. Da Silveira, "Evaluation of recombinant antigens for serodiagnosis of Chagas' disease in South and Central America," J. Clin. Microbiol., 37, 1554-1560 (1999).
Received: November 10, 2011.
Accepted: April 18, 2012
Recommended by subject editor: Pedro de Alcântara Pessôa












