Serviços Personalizados
Artigo
Latin American applied research
versão On-line ISSN 1851-8796
Lat. Am. appl. res. vol.43 no.1 Bahía Blanca jan. 2013
Production of struvite as an alternative to reduce the content of nitrogen and phosphorus from swine wastewater
E.L. Foletto†*, W.R.B. dos Santos†, S.L. Jahn†, M.A. Mazutti†, R. Hoffmann†, A. Cancelier‡ and E. Müller‡
† Department of Chemical Engineering,
‡ Department of Chemistry, Federal University of Santa Maria, Santa Maria-RS, 97105-900, Brazil.
* E-mail: efoletto@gmail.com
Abstract— This work is focused on the production of struvite as an alternative to reduce the concentration of phosphorus and nitrogen from swine wastewater. The effect of Mg source (MgO and MgCl2. 6H2O) and pH on nitrogen and phosphorus removal was investigated. From the results was seen that N and P were efficiently removed from swine wastewater. Data of X-ray diffraction (XRD) revealed that the production of struvite in the crystalline form is dependent of pH, which was obtained only in the runs carried out at pH 9.5, independent of Mg source employed. The production of struvite showed to be effective for adding value to swine wastewater.
Keywords— Struvite; Wastewater; Nitrogen; Phosphorous.
I. INTRODUCTION
Swine wastewater contains high concentration of phosphorus (P) and nitrogen (N), and the removal of these nutrients from wastewater is important to maintain the water quality, avoiding the eutrophication (Haddrill et al., 1983; O'Hare et al., 2010). Ammonia in swine was-tewater is usually removed by biological methods such as autotrophic nitrification (conversion of 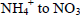 ) (Furtado et al., 1998; Iliuta et al., 2002; Queiroz et al., 2011) and heterotrophic denitrification (conver-sion of NO3- to gaseous nitrogen) (Reginatto et al., 2005; Garbossa et al., 2005; Canto et al., 2008). However, the struvite precipitation method is interesting because it simultaneously removes and recovers P and N from wastewater (Liu et al., 2011). Struvite is a crystalline solid with equal molar concentrations of magnesium, ammonium and phosphorus (MgNH4PO4.6H2O). The formation of struvite normally occurs in alkaline medium. The optimal pH value for struvite crystallization was considered to be 8.0-11.0 (Laridi et al., 2005; Hanhoun et al., 2011). Struvite is used as a slow-release fertilizer (Bridger et al., 1962). Therefore, the production of struvite is an alternative for adding value to swine wastewater, besides to decrease the impact caused by its disposal into the environment (Nelson et al., 2003).
) (Furtado et al., 1998; Iliuta et al., 2002; Queiroz et al., 2011) and heterotrophic denitrification (conver-sion of NO3- to gaseous nitrogen) (Reginatto et al., 2005; Garbossa et al., 2005; Canto et al., 2008). However, the struvite precipitation method is interesting because it simultaneously removes and recovers P and N from wastewater (Liu et al., 2011). Struvite is a crystalline solid with equal molar concentrations of magnesium, ammonium and phosphorus (MgNH4PO4.6H2O). The formation of struvite normally occurs in alkaline medium. The optimal pH value for struvite crystallization was considered to be 8.0-11.0 (Laridi et al., 2005; Hanhoun et al., 2011). Struvite is used as a slow-release fertilizer (Bridger et al., 1962). Therefore, the production of struvite is an alternative for adding value to swine wastewater, besides to decrease the impact caused by its disposal into the environment (Nelson et al., 2003).
Struvite has been obtained from industrial wastewater (Diwani et al., 2007), leather tanning wastewater (Tunay et al., 1997), waste sludge (Jaffer et al., 2002), poultry wastewater (Yetilmezsoy and Zengin, 2009), swine wastewater (Song et al., 2011; Rahman et al., 2011) and municipal landfill leachate (Kim et al., 2007). Although there are many works in literature reporting the phosphorus and nitrogen removal by struvite precipitation method from swine, it is difficult to found studies reporting/comparing different sources of Mg on the formation of struvite (Li et al., 1999; Li and Zhao, 2003; Demirer et al., 2005; Huang et al., 2011), since the characteristic of material formed as well as the recovering efficiency can be influenced by this variable.
In this sense, the effect of the magnesium source and pH on the removal of phosphorus and nitrogen by struvite crystallization method from swine wastewater was investigated. The struvite obtained was analyzed by X-ray diffraction (XRD) and infrared spectroscopy (IR).
II. METHODS
The swine wastewater sample used in this work was collected from a local farm. The sample was stored and maintained at 4°C until analysis. The sample was centrifuged at 3000 rpm for 15 min to separate the solids, and the supernatant was used for chemical analysis. The characteristics of the swine wastewater are summarized in Table 1.
Table 1: Characteristics of the swine wastewater.

The system consisted of a glass-batch reactor (Jar test) (11x11x17 cm) with a total volume of 2.0 L, constituted of a paddle of diameter 7.5 cm and height of 2.5 cm. The working volume of the reactor was 1.0 L. The reactor was operated under agitation of 200 rpm, at room temperature (25oC). Analytical grade chemicals (MgO, MgCl2.6H2O, H3PO4 (85%), KH2PO4 and NaOH) were used in the experiments. It was evaluated the synthesis of struvite using two different Mg sources (MgO and MgCl2.6H2O) and at two different pH (9.0 and 9.5). In addition, two combinations of chemicals, MgO + H3PO4 (4M) and MgCl2.6H2O + KH2PO4, were used in the experiments. The pH of solutions was adjusted by adding NaOH 4M. The amounts of MgO + H3PO4 (4M) and MgCl2.6H2O+KH2PO4 used in the tests were calculated according to the concentration of nitrogen and phosphorous of the swine wastewater sample (see Table 1) in a molar ratio of 1:1:1 (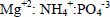 ). All runs were carried out by 10 min, and then kept at rest for 30 min. The suspensions were filtered and the deposited solids were washed thorough-ly with distilled water at room temperature and dried at 50oC for 8 h. The analytical tests performed in the supernatant were
). All runs were carried out by 10 min, and then kept at rest for 30 min. The suspensions were filtered and the deposited solids were washed thorough-ly with distilled water at room temperature and dried at 50oC for 8 h. The analytical tests performed in the supernatant were 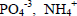 , pH and total chemical oxygen demand (TCOD). The nitrogen content was determined by a colorimetric method using the Nessler reagent (APHA, 1999). The dissolved phosphorus concentration was determined by ascorbic acid colorimetric method (Perera et al., 2009). TCOD was analyzed according to the Standard Methods (APHA, 1999).
, pH and total chemical oxygen demand (TCOD). The nitrogen content was determined by a colorimetric method using the Nessler reagent (APHA, 1999). The dissolved phosphorus concentration was determined by ascorbic acid colorimetric method (Perera et al., 2009). TCOD was analyzed according to the Standard Methods (APHA, 1999).
The precipitated struvite was analyzed by X-ray diffraction (XRD) and infrared spectroscopy (IR). The precipitated crystals were indentified using an X-ray Diffractometer (Philips, MPD 1880 model), where the X-ray source was Cu-Kα radiation, powered at 40 kV and 40 mA. The average nanocrystals size was determined through X-ray diffraction (and reflection) line broadening using the Sherrer equation: D= K.λ/ (β.cosθ), where D is the crystallite size, K is the Sherrer constant (0.90), λ is the wavelength of the X-ray radiation (0.1542495 nm for Cu-Kα), and β is the peak width at half height and finally θ corresponds to the peak position (in the current study, 2θ= 20.84). IR spectra were recorded on a PerkinElmer FT-IR Spectrum spectrophotometer in the region of 440-4000 cm-1, using KBr pellets. Mg2+, Ca2+ and Na+ were analyzed by a Hitachi atomic absorption spectrometer.
III. RESULTS AND DISCUSSION
Table 1 shows the characteristics of the swine wastewater used in this study. As can be seen, the sample present high amount of nitrogen and phosphorus, which were 824 and 705 mg.L-1, respectively. The sample also has high TCOD content, 360 mgO2.L-1.
Figure 1 shows the results concerning the removal of 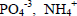 and TCOD obtained in experimental runs carried out in this work. The efficiency of removal (%) of
and TCOD obtained in experimental runs carried out in this work. The efficiency of removal (%) of 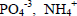 and TCOD (%) was obtained by equation: Percentage Removal (%)=[Ctotal(i) - Ctotal(o)]/ Ctotal(i), where Ctotal(i) and Ctotal(o) are the
and TCOD (%) was obtained by equation: Percentage Removal (%)=[Ctotal(i) - Ctotal(o)]/ Ctotal(i), where Ctotal(i) and Ctotal(o) are the 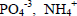 and TCOD concentrations before and after the reaction.
and TCOD concentrations before and after the reaction.
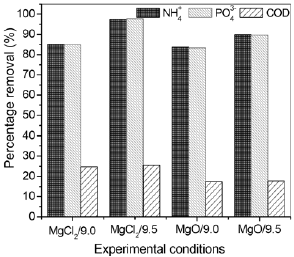
Fig. 1. Percentage removal of 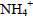 ,
, 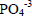 and TCOD after the reaction at different experimental conditions.
and TCOD after the reaction at different experimental conditions.
As observed in Fig. 1, the Mg source influenced the amount of 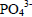 ,
, 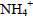 and TCOD removed during the struvite precipitation. The removal of
and TCOD removed during the struvite precipitation. The removal of 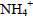 and
and 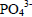 was higher using MgCl2.6H2O than MgO in both pH. At pH 9.5 was recovered about 95% and 90% using MgCl2.6H2O and MgO, respectively, for both
was higher using MgCl2.6H2O than MgO in both pH. At pH 9.5 was recovered about 95% and 90% using MgCl2.6H2O and MgO, respectively, for both 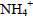 and
and 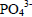 . Concerning the effects of pH, it was verified that at pH 9.5 was obtained better results, since the amount of
. Concerning the effects of pH, it was verified that at pH 9.5 was obtained better results, since the amount of 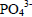 and
and 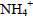 recovered was slightly higher than pH 9.0. This variation was more pronounced using MgCl2.6H2O as Mg source. The best result obtained using MgCl2.6H2O can be explained due to its quick dissociative nature (Liu et al., 2008). Song et al. (2011) observed reductions of
recovered was slightly higher than pH 9.0. This variation was more pronounced using MgCl2.6H2O as Mg source. The best result obtained using MgCl2.6H2O can be explained due to its quick dissociative nature (Liu et al., 2008). Song et al. (2011) observed reductions of 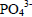 and
and 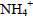 about 85% and 40-90% by struvite formation in swine wastewater, respectively. Recovery ranging from 55 to 98% of
about 85% and 40-90% by struvite formation in swine wastewater, respectively. Recovery ranging from 55 to 98% of 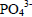 and 36 to 50% of
and 36 to 50% of 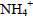 in the treatment of liquid swine manure has been obtained (Çelen et al., 2007). Results of
in the treatment of liquid swine manure has been obtained (Çelen et al., 2007). Results of 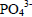 reduction about 85% by struvite precipitation in anaerobic swine lagoon liquid has been reported (Nelson et al., 2003). Concerning the results of TCOD, was not verified significant reduction in their values (about 25%) after the treatment. However, the results obtained in this study are in good agreement with those reported by Li et al. (1999), which reported a TCOD reduction about 30% in treating landfill leachate effluent. They suggested that after struvite precipitation, a biological treatment process can be accomplished to remove COD. This implies that the chemical precipitation technique has a significant selectivity to remove phosphorous and nitrogen from wastewater. Results of TCOD reduction of about 47% in treating swine wastewater were reported (Ryu and Lee, 2010). Ozturk et al. (2003) reported that COD removal of about 50% in treating anaerobically pre-treated raw landfill leachate effluent by struvite crystallization. The amount of Ca2+, Na+ and Mg2+ were quantified only for the swine wastewater treated with MgCl2.6H2O at pH 9.5. The amount of Ca2+ and Na+ in swine wastewater treated were 64 and 128 (mg.L-1), respectively. Thus, concentrations of Ca2+ and Na+ were significantly reduced (61% and 60%, respectively) during the struvite precipitation process. The results indicated that these elements were co-precipitated along with the struvite during the crystallization process. Meanwhile, the concentration of Mg2+ increased after treatment (15 mg.L-1), and this may be due the addition of MgCl2.6H2O into the reaction system as Mg2+ source. Similar behaviors have been reported by Liu et al. (2011).
reduction about 85% by struvite precipitation in anaerobic swine lagoon liquid has been reported (Nelson et al., 2003). Concerning the results of TCOD, was not verified significant reduction in their values (about 25%) after the treatment. However, the results obtained in this study are in good agreement with those reported by Li et al. (1999), which reported a TCOD reduction about 30% in treating landfill leachate effluent. They suggested that after struvite precipitation, a biological treatment process can be accomplished to remove COD. This implies that the chemical precipitation technique has a significant selectivity to remove phosphorous and nitrogen from wastewater. Results of TCOD reduction of about 47% in treating swine wastewater were reported (Ryu and Lee, 2010). Ozturk et al. (2003) reported that COD removal of about 50% in treating anaerobically pre-treated raw landfill leachate effluent by struvite crystallization. The amount of Ca2+, Na+ and Mg2+ were quantified only for the swine wastewater treated with MgCl2.6H2O at pH 9.5. The amount of Ca2+ and Na+ in swine wastewater treated were 64 and 128 (mg.L-1), respectively. Thus, concentrations of Ca2+ and Na+ were significantly reduced (61% and 60%, respectively) during the struvite precipitation process. The results indicated that these elements were co-precipitated along with the struvite during the crystallization process. Meanwhile, the concentration of Mg2+ increased after treatment (15 mg.L-1), and this may be due the addition of MgCl2.6H2O into the reaction system as Mg2+ source. Similar behaviors have been reported by Liu et al. (2011).
The identification of powders was examined by XRD (Fig. 2), which indicated the formation of amorphous solid for the samples synthesized at pH 9.0, independent of Mg source. By other hand, at pH 9.5, it was verified the formation of crystalline struvite for both sources of Mg. Several authors have been reported that optimum pH range for formation of struvite is very narrow and it is dependent of the quality of raw material. In previous works concerning struvite precipitation of swine wastewater, the optimum pH of 8.0-8.5 (Huang et al., 2011), 8.5 (Suzuki et al., 2002), 9.0 (Jaffer et al., 2002), 8.9-9.25 (Nelson et al., 2003), 9.5-10.5 (Song et al., 2007) were reported. If the pH is maintained at values above the optimum range occurs the formation of Mg3(PO4)2 instead of struvite, whereas below this range promotes the increase of H+ in the solution inhibiting the struvite crystallization (Huang et al., 2011).
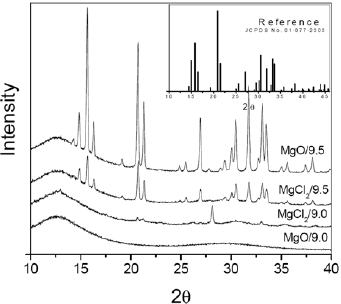
Fig 2. XRD analysis of samples obtained in different pH and Mg sources and (insert) reference struvite (JCPDS Card No 1-077-2303).
The formation of struvite is confirmed by location of peaks, corresponding to reference database lines for struvite (see insert in Fig. 2).
From Fig. 2 no significant differences appear on position of peaks among the samples obtained from different Mg sources, at pH 9.5. By applying the Scherrer equation, the average nanocrystal domain sizes were estimated to be 63 and 69 nm, for the samples obtained at pH 9.5 using MgO and MgCl2.6H2O as Mg source respectively. It is important to point out that the amorphous solid obtained at pH 9.0 was effective to remove 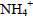 and
and 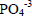 from swine wastewater, presenting values of removal higher than 80% for both contaminants.
from swine wastewater, presenting values of removal higher than 80% for both contaminants.
The identification of struvite phase was confirmed by IR spectroscopy (Fig. 3). The samples obtained using MgO and MgCl2.6H2O, at pH 9.5, showed a band at 1440 cm-1 that is characteristic of struvite (Babić-Ivančić et al., 2006). The first four bands observed about 3600, 3500, 3260 and 3115 cm-1 belong to the OH stretching vibrations. The band at 2970 cm-1 was the anti-symmetric stretching vibration of 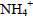 groups. The broad band between 2500 and 2200 cm-1 was assigned to water-phosphate H-bonding. HOH deformation of water was at 1680 cm-1, and the bands seen over the range of 1600 to 1400 cm-1 were those of the HNH deformation modes of HN4. The bands of
groups. The broad band between 2500 and 2200 cm-1 was assigned to water-phosphate H-bonding. HOH deformation of water was at 1680 cm-1, and the bands seen over the range of 1600 to 1400 cm-1 were those of the HNH deformation modes of HN4. The bands of 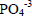 units were observed at 1006, 571, 463 and 438 cm-1. Water-water H bonding was observed at 760 and 695 cm-1, whereas ammonium-water H bonding was observed at 890 cm-1 (Kurtulus and Tas, 2011).
units were observed at 1006, 571, 463 and 438 cm-1. Water-water H bonding was observed at 760 and 695 cm-1, whereas ammonium-water H bonding was observed at 890 cm-1 (Kurtulus and Tas, 2011).
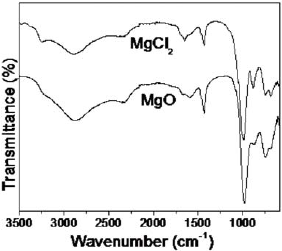
Fig.3. IR bands of struvite samples obtained from MgO and MgCl2.6H2O, at pH 9.5.
IV. CONCLUSIONS
In this work results concerning the removal of 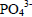 and
and 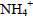 from swine wastewater by struvite crystallization process were presented. Data of X-ray diffraction (XRD) revealed that the production of struvite in the crystalline form depends on pH. Struvite was obtained only in the runs carried out at pH 9.5, independently of the Mg source employed. In addition, the amorphous material obtained at pH 9.0 was effective to recover
from swine wastewater by struvite crystallization process were presented. Data of X-ray diffraction (XRD) revealed that the production of struvite in the crystalline form depends on pH. Struvite was obtained only in the runs carried out at pH 9.5, independently of the Mg source employed. In addition, the amorphous material obtained at pH 9.0 was effective to recover 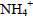 and
and 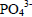 , since a reduction higher than 80% was verified. The production of struvite showed to be effective for adding value to swine wastewater.
, since a reduction higher than 80% was verified. The production of struvite showed to be effective for adding value to swine wastewater.
REFERENCES
1. APHA - American Public Health Association. Standard methods for the examination of water and wastewater. American Water Works Association, and Water Pollution, Control Federation. 19th ed., Washington, D.C. (1999).
2. Babić-Ivančić, V., J. Kontrec, L. Brečević and D. Kralj, "Kinetics of struvite to newberyite transformation in the precipitation system MgCl2-NH4H2PO4-NaOH-H2O," Water Res., 40, 3447-3455 (2006).
3. Bridger, G.L., M.L. Salutsky and R.W. Starostka, "Metal ammonium phosphates as fertilizers," Agric. Food Chem., 10, 181-188 (1962).
4. Canto, C.S.A., S.M. Ratusznei, J.A.D. Rodrigues, M. Zaiat and E. Foresti, "Effect of ammonia load on efficiency of nitrogen removal in an SBBR with liquid-phase circulation," Braz. J. Chem. Eng., 25, 275-289 (2008).
5. Çelen, I., J. Buchanan, R. Burns, R. Robinson and D. Raman, "Using a chemical equilibrium model to predict amendments required to precipitate phosphorus as struvite in liquid swine manure," Water Res., 41, 1689-1696 (2007).
6. Demirer, S.U., G.N. Demirer and S. Chen, "Ammonia removal from anaerobically digested dairy manure by struuvite precipitation," Proc. Biochem., 40, 3667-3674 (2005).
7. Diwani, G., S. Rafie, N.N. Ibiari and H.I. El-Aila, "Recovery of ammonia nitrogen from industrial wastewater treatment as struvite slow releasing fertilizer," Desalination, 214, 200-214 (2007).
8. Furtado, A.A.L., R.T. Albuquerque, S.G.F. Leite and R.P. Pecanha, "Effect of hydraulic retention time on nitrification in an air Lift biological reactor," Braz. J. Chem. Eng., 15, 303-307 (1998).
9. Garbossa, L.H.P., K.R. Lapa, M. Zaiat and E. Foresti, "Development and evaluation of a radial anaerobic/aerobic reactor treating organic matter and nitrogen in sewage," Braz. J. Chem. Eng., 22, 511-519 (2005).
10. Haddrill, V., R. Keffer, G.C. Olivetti, G.B. Poller and F. Giovanardi, "Eutrophication problems in Emilia Romagna, Italy: monitoring the nutrient load discharged to the littoral zone of the Adriatic Sea," Water Res., 17, 483-495 (1983).
11. Hanhoun, M., L. Montastruc, C. Azzaro-Pantel, B. Biscans, M. Frèche and L. Pibouleau, "Temperature impact assessment on struvite solubility product: A thermodynamic modeling approach," Chem. Eng. J., 167, 50-58 (2011).
12. Huang, H., C. Xu and W. Zhang, "Removal of nutrients from piggery wastewater using struvite precipitation and pyrogenation technology," Biores. Techn., 102, 2523-2528 (2011).
13. Iliuta, I., S.C. Bildea, M.C. Iliuta and F. Larachi, "Analysis of trickle bed and packed bubble column bioreactors for combined carbon oxidation and nitrification," Braz. J. Chem. Eng., 19, 69-88 (2002).
14. Jaffer, Y., T.A. Clark, P. Pearce and S.A. Parsons, "Potential phosphorus recovery by struvite formation," Water Res., 36, 1834-1842 (2002).
15. Kim, D., H. Ryu, M. Kim, J. Kim and S. Lee, "Enhancing struvite precipitation potential for ammonia nitrogen removal in municipal landfill leachate," J. Hazar. Mat., 146, 81-85 (2007).
16. Kurtulus, G. and A.C. Tas, "Transformations of neat and heated struvite (MgNH4PO4.6H2O)," Mater. Lett., 65, 2883-2886 (2011).
17. Laridi, R., J. Auclair and H. Benmoussa, "Laboratory and pilot-scale phosphate and ammonium removal by controlled struvite precipitation following coagulation and flocculation of swine wastewater," Environm. Techn., 26, 525-536 (2005).
18. Li, X.Z and Q.L. Zhao, "Recovery of ammonium-nitrogen from landfill leachate as a multi-nutrient fertilizer," Ecological Eng., 20, 171-181 (2003).
19. Li, X.Z., Q.L. Zhao and X.D. Hao, "Ammonium removal from landfill leachate by chemical precipitation," Waste Manag., 19, 409-415 (1999).
20. Liu, Y., J. Kwag, J. Kim and C. Ra, "Recovery of nitrogen and phosphorus by struvite crystallization from swine wastewater," Desalination, 277, 364-369 (2011).
21. Liu, Z.G., Q.L. Zhao, D.J. Lee and N. Yang, "Enhancing phosphorous recovery by a new internl recycle seeding MAP reactor," Bioresour. Technol., 99, 6488-6493 (2008).
22. Nelson, N.O., R.L. Mikkelsen and D.L. Hesterberg, "Struvite precipitation in anaerobic swine lagoon liquid: effect of pH and Mg:P ratio and determination of rate constant," Biores. Techn., 9, 229-236 (2003).
23. O'Hare, M.T., R.T. Clarke, M.J. Bowes, C. Cailes, P. Henville, N. Bissett, C. McGahey and M. Neal, "Eutrophication impacts on a river macrophyte," Aquatic Botany, 92, 173-178 (2010).
24. Ozturk, K.I., M. Altinbas, I. Koyuncu, O. Arikan and C. Yangin, "Advanced physico-chemical treatment experiences on young municipal landfill leachates," Waste Manag., 23, 441-446 (2003).
25. Perera, P.W.A., W.X. Wu, Y.X. Chen and Z.Y. Han, "Struvite recovery from swine waste biogas digester effluent through a stainless steel device under constant pH conditions," Biomedical and Environm. Sci., 22, 201-209 (2009).
26. Queiroz, L.M., M.V. Aun, D.M. Morita and P.A. Sobrinho, "Biological nitrogen removal over nitritation/denitritation using phenol as carbon source," Braz. J. Chem. Eng., 28, 197-207 (2011).
27. Rahman, M.M., Y. Liu, J. Kwag and C. Ra, "Recovery of struvite from animal wastewater and its nutrient leaching loss in soil," J. Hazar. Mater., 186, 2026-2030 (2011).
28. Reginatto, V., R.M. Teixeira, F. Pereira, W. Schmidell, A. Furigo Jr., R. Menes, C. Etchebehere and H.M. Soares, "Anaerobic ammonium oxidation in a bioreactor treating slaughterhouse wastewater," Braz. J. Chem. Eng., 22, 593-600 (2005).
29. Ryu, H. and S. Lee, "Application of struvite precipitation as a pretreatment in treating swine wastewater," Proc. Biochem., 45, 563-572 (2010).
30. Song, Y., G. Qiu, P. Yuan, X. Cui, J. Peng, P. Zeng, L. Duan, L. Xiang and F. Qian, "Nutrients removal and recovery from anaerobically digested swine wastewater by struvite crystallization without chemical additions," J. Hazar. Mater., 190, 140-149 (2011).
31. Song, Y., P. Yuan, B. Zheng, J. Peng, F. Yuan and Y. Gao, "Nutrients removal and recovery by crystallization of magnesium ammonium phosphate from synthetic swine wastewater," Chemosphere, 69, 319-324 (2007).
32. Suzuki, K., Y. Tanaka, T. Osada and M. Waki, "Removal of phosphate, magnesium and calcium from swine wastewater through crystallization enhanced by aeration," Water Res., 36, 2991-2998 (2002).
33. Tunay, O., I. Kabdasli, D. Orhon and S. Kolçak, "Ammonia removal by magnesium ammonium phosphate precipitation in industrial wastewaters," Water Sci. Tech., 36, 225-228 (1997).
34. Yetilmezsoy, K and Z. Zengin, "Recovery of ammonium nitrogen from the effluent of UASB treating poultry manure wastewater by MAP precipitation as a slow release fertilizer," J. Hazar. Mater., 166, 260-269 (2009).
Received: December 16, 2011.
Accepted: June 18, 2012.
Recommended by Subject Editor María Luján Ferreira.