Servicios Personalizados
Articulo
Latin American applied research
versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.43 no.4 Bahía Blanca oct. 2013
Photocatalytic degradation of 2-chlorophenol by tio2: kinetic studies
G.V. Morales†, E.L. Sham†, R. Cornejo† and M.E. Farfan Torres‡
† Facultad de Ingeniería, INIQUI-CIUNSa, Univ. Nac. de Salta, 4400 Salta, Argentina.
gmorales@unsa.edu.ar, sham@unsa.edu.ar
‡ Facultad de Ciencias Exactas, Univ. Nac. de Salta, 4400 Salta, Argentina. mfarfan@unsa.edu.ar
Abstract— Kinetic studies of 2-chlorophenol photocatalytic degradation are carried out in a batch stirred built in quartz laboratory scale, using TiO2 as catalyst photoactived with ultraviolet light. Experimental design is performed using as independent variables or factors: catalyst concentration, catalyst calcinations temperature and initial concentration of 2-chlorophenol, to establish the best conditions of the degradation process. The experimental data were fitted with the Langmuir-Hinshelwood model. A kinetic constant k of 0.24 mg L-1min-1 was obtained.
Keywords— 2-chlorophenol; Photocatalysis; Kinetics.
I. INTRODUCTION
Photocatalysis is a particular case of heterogeneous catalysis. This process is based on the direct or indirect adsorption by a solid, usually a semiconductor, of photons of visible or UV light. When illuminated, this semiconductor, owing to its electronic structure, can generate electron-hole pairs by promoting an electron from the valence band to the conduction band, thus leaving a gap in the latter band. These holes can absorb H2O or hydroxyls from the reaction medium and produce highly reactive species of hydroxyl radicals. In addition, the electrons promoted to the conduction band can reduce the molecular oxygen to peroxide anion to form hydrogen peroxide or organic peroxides in the presence of organic compounds (Akpan and Hameed, 2009). Radical hydroxyls are strong oxidizing agents that can degrade the organic compounds or their intermediaries to reach final products such as CO2, H2O, N2. Photocatalysis can thus be defined as the acceleration of a chemical reaction by the presence of a catalyst. When the catalyst is activated by light adsorption, it accelerates the process by interacting with the reactive by means of the appearance of electron-hole (e-, h+) pairs, which is a characteristic of semiconductor materials.
TiO2 is the most widely used semiconductor in photocatalysis, because it is chemically and biologically inert and is stable to photochemical and chemical corrosion. It is also the most widely used, because of its nontoxic nature, availability and low cost (Linsebigler et al., 1995). In addition, the TiO2 has a gap energy of 3.2 eV and can be excited with UV light λ < 387 nm, which may be provided by a small fraction of the spectrum of sunlight.
TiO2 is found in three crystalline forms: brookite, rutile and anastasa, being the last two the most effective for photocatalytic degradation of organic compounds. Anastasa is thermodynamically less stable than rutile. However, it has more surface area and high surface density of active sites for adsorption and catalysis. The energies of the gap are 3.2 eV and 3.0 eV for anastase to rutile respectively. Nevertheless, oxidative processes are similar.
Chlorophenols represent an important class of very common water pollutants. Because of their extensive use as fungicides, herbicides and wood preservatives (Paasivirta, 1998), they can easily be found in soils and in aquatic environments. Others sources include the waste incineration or disinfection of sewage and industrial wastewater with chlorine, as well as discharges from paper mills, releasing them as by-products of chlorine-based bleaching (Krijgsheld and Van der Gen, 1986).
Chlorophenols are persistent water pollutants under environmental conditions, due to the stability of the C-Cl bond, which is also responsible for their toxicity (Roques, 1996). Most of them have been listed as toxic or priority pollutants by both the US Environmental Protection Agency (EPA).
In relation to the kinetic modeling of photocatalytic reactions, Peiró et al. (2001) studied the photocatalytic degradation of a solution containing a mixture of four organic compounds: phenol, guaiacol, 2-chloro-phenol and catechol in aqueous suspensions of TiO2. They showed that the photodegradation of the different organic compounds follows the Langmuir-Hinshelwood kinetic model and the reaction rate decreases in the following order: guaiacol > 2-chlorophenol ≈ phenol > catechol. Moreover, Bekbolet et al. (2002) in their studies of humic acid photocatalytic degradation, performed the kinetic analysis in terms of a pseudo-first order kinetics (at low substrate concentrations) and the Langmuir-Hinshelwood kinetics (at high substrate concentrations), showing that the reaction rate does not depend on the specific surface of the catalyst. They also showed that the morphological and crystallographic properties of the TiO2 can play an important role in photocatalytic efficiency. Sobczynski et al. (2004) studied the photocatalytic decomposition of phenol by illuminated TiO2. They recommend the use of the initial reaction rate for kinetic studies, due to the existence of many competing reactions in the suspensions of illuminated TiO2. They also present a kinetic mechanism for the complete mineralization of phenol. Wu et al. (2006) studied the dye decomposition kinetics by nano-sized TiO2 suspension by varying the agitation speed, TiO2 suspension concentration, initial dye concentration, temperature, and UV power intensity. They developed a kinetic model, based on the Langmuir-Hinshelwood model and the Lambert-Beer's law, to successfully correlate the initial rates. Furthermore, Gondal et al. (2007) applied photocatalysis to compare the catalytic activity of four photocatalysts in phenol degradation in water, by irradiating with laser: WO3, NiO, Fe2O3 and TiO2. The maximum removal of phenol was achieved with WO3. The reaction rates for different catalysts were 0.0103, 0.0081, 0.0134 and 0.0169 min-1 for WO3, TiO2, NiO and Fe2O3 respectively. The degradation process follows a first order kinetics. Moreover, Farias et al. (2009) focused their study on the kinetic modeling of the Fenton and photo-Fenton degradation of a model pollutant (formic acid) in aqueous solution, for low iron concentrations. They derived the reaction rate expressions from an accepted reaction mechanism and explicitly taking into account the local volumetric rate of photon adsorption. Friedmann et al. (2010) discussed the relevance of the parameters affecting the kinetics and mechanisms of photocatalysis for photocatalytic treatment of water by using TiO2. They determined that there is a strong interplay between pollutant structure, reactivity, and mode of interaction with catalyst surface. They also determined that for each pollutant, a unique set of conditions may be needed for optimal performance. Zhang et al. (2011) studied the photocatalytic degradation kinetics of rhodamine B (RhB) by TiO2-coated activated carbon (TiO2/AC) catalyst under different reaction conditions (light intensity, TiO2 content in TiO2/AC and initial content of contamination). They found that the kinetics of RhB photodegradation follows the first-order rate law and could be described by a modified Langmuir-Hinshelwood model.
The importance of this paper derives from the fact that there are few reports related to kinetic studies of photocatalytic degradation of 2-chlorophenol (Wang et al., 1999). Consequently, the objective of this paper is to study the kinetic of photocatalytic degradation of 2-chlorophenol, by analyzing the effects of factors such as catalyst concentration, calcination temperature of the catalyst and initial concentration of 2-chlorophenol, to establish the best conditions of the degradation process. The following kinetic parameters were determined: kinetic constant (k) and adsorption constant (K).
II. METHODS
A. Experimental Procedure
Photocatalytic degradation of 2-chlorophenol was carried out in a quartz cylindrical batch stirred reactor with a capacity of 150 cm3, illuminated laterally with a 300 W lamp, an OsramUltra Vitalux.
The catalyst was prepared by means of the sol-gel technique, using titanium isopropoxide (Aldrich 97%) as precursor. Hydrolysis of a 1M solution of titanium isopropoxide in isopropyl alcohol (Ciccarelli P.A) was carried out, using ultrapure H2O, acidulated with HNO3 (pH=2.5) as hydrolyzing media. The 1M solution of titanium isopropoxide was slowly added to the constantly stirred hydrolysis media. The H2O/Ti ratio used was 4. The addition of alcoxide produced the formation of titanium oxohydroxide, denoted by the formation of a whitish dispersion in the reactive medium. This suspension was stirred for 24 hours and the solid was then separated and washed by centrifugation and drying at 65 °C.
The 2-chlorophenol solution and the photocatalyst were kept agitated by a magnetic stirring bar located at the bottom of the reactor, while the experiment lasted. The reaction temperature was kept constant at 35°C.
B. Experimental Design
Preliminary experiments were conducted to determine whether the sample could be degraded by the photocatalytic process and to determine the levels of catalyst concentration, reaction time and initial concentration of organic compound. Two types of experiments on blank were carried out, one non-illuminated with the addition of TiO2 and another in absence of TiO2 with illumination of the 2-chlorophenol solution. The following factors (independent variables) were selected to programme the experiments: concentration of the catalyst (Cs), calcination temperature of the catalyst (Tcal) and initial concentration of the 2-chlorophenol (Co). Table 1 shows the levels of the factors used in the experiments.
Table 1: Factors and their values used in the experiments

The stirring speed was kept constant at 600 rpm after verifying that it has no significant effect on the degradation rate of the organic matter. The pH of the solution remained constant with an approximate value of 5 during the whole experiment.
X was chosen as the only system response to represent the amount of degradation that experiences the organic compound with regard to its initial concentration, Co, and was defined as:
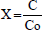 | (1) |
where C is the concentration of the dissolved organic compound in the solution at the given time. The definition of X given by the Eq. (1), was the one used to follow the course of the organic compound degradation. To this end, at pre-programmed reaction intervals, samples were taken from the reaction mixture, centrifuged to separate the catalyst and analyzed by means of UV spectrophotometry at a wavelength of 275 nm.
C. Results and discussion
Effect of the concentration of TiO2
Figure 1 shows the effect of the concentration of the photocatalyst (TiO2), calcined at 500°C, on the 2-chlorophenol degradation.
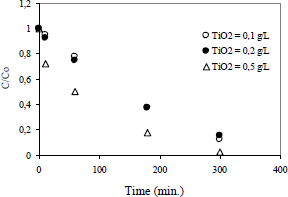
Figure 1. Effect of the photocatalyst concentration
The effect of photocatalyst concentration begins to become important when the concentration of the solid increases significantly. For example, when the concentration of the photocatalyst increases by five times (Cs=0.1 g/L to Cs = 0.5 g/L), the degradation of the organic compound increases by 60% after 300 minutes of starting the reaction.
Effect of the calcination temperature of the TiO2
The influence of the calcination temperature of TiO2 on the degradation of the organic substance, at a TiO2 concentration of 0.1 g/L is shown in the Fig. 2.
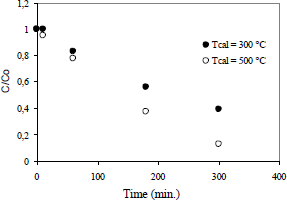
Figure 2. Effect of the calcination temperature of the photocatalyst
It can be observed that increasing the calcination temperature of TiO2 has a significant influence on the degradation of the organic compound. For example, at a calcination temperature to 200°C, the degradation of organic compound increased by 70% after 300 minutes of starting the reaction. It is thus clear that the crystallinity of the anastase phase increases in line with the calcination temperature of TiO2. The XRD patterns at 300 °C and 500 °C for all solids correspond to pure anatase. The anatase structure was also confirmed by Raman spectroscopy. From XRD analysis, it can also be observed that the crystallization of anatase phase increased with the increase of heat-treatments temperature from 300 °C to 500 °C showing a profile of sharp diffraction peak (101) at a higher treatment temperature.
Effect of the initial concentration of the organic compound
In order to analyze the influence of the initial concentration of the 2-chlorophenol on its degradation rate, the data are plotted in the Fig. 3, for a TiO2 concentration of 0.2 g/L, calcined at 500°C.
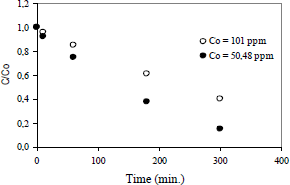
Figure 3. Effect of the initial concentration of the organic compound
It is to be noted that increasing the initial concentration of the organic compound, the degree of degradation rate decreases for a given time. This agrees, essentially, with the general theoretical aspects of photocatalytic reactions.
D. Kinetic Modeling
The kinetics of the photocatalytic degradation of many organic compounds in suspensions of TiO2 under illumination has been modeled using the equation of Langmuir-Hinshelwood (L-H) (Valente et al., 2006). This model considers that the reaction rate is proportional to the photocatalyst surface fraction covered by the substrate (θ).
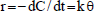 | (2) |
with
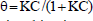 | (3) |
where k is the reaction kinetic constant and K is the constant of the reactant adsorption on the particles of TiO2.
Substituting Eq. (2) in Eq. (3) becomes:
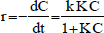 | (4) |
Langmuir-Hinshelwood equation (L-H) models a reaction mechanism involving adsorption equilibrium and a slow surface reaction. This equation can be linearized to fit the experimental data and find the kinetic constants, using the substrate concentration curves, C, versus time, t, to calculate the actual reaction rate, r.
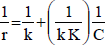 | (5) |
From the L-H kinetic expression, Eq. (5), the values of the rate constant, k, and the adsorption constant, K, are determines. These values are shown in Table 2.
Table 2: Kinetic constant and adsorption constant of the phenol degradation
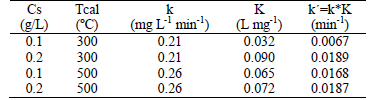
From the analysis of the data presented in Table 2, it is possible to infer that the kinetic constant k is equal to approximately 0.24 mg L-1 min-1. This is consistent with studies by Bekbolet et al. (2002), who show that the photocatalytic rate is not necessarily dependent on the specific surface of the photocatalyst. They also showed that the morphological and crystallographic properties of the photocatalyst play an important role in photocatalytic efficiency.
To test the agreement between the experimental data and the values calculated from the mathematical model, the graph of Cexp versus Cteo was plotted as shown in Fig. 4, for a photocatalyst concentration of 0.2 g/L, calcined at 300°C and at 500°C. It is observed that the agreement between the experimental and the calculated values is very good.
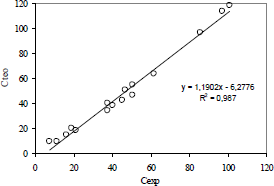
Figure 4. Agreement between experimental and calculated 2-chlorophenol concentration values.
According to Fig. 4, it can be seen that the data show a good correlation coefficient, 0.987, thus indicating an excellent approximation to the L-H model.
III. CONCLUSIONS
The heterogeneous photocatalysis is an alternative for the degradation of organic compounds such as 2-chlorophenol. The degradation process shows a good efficiency although the rate is slow. Increasing the initial concentration of the photocatalyst and its calcination temperature increases the degradation rate, being the calcinations temperature the most significant effect. When increasing the initial concentration of 2-chloro-phenol, the degree of its degradation decreases. This goes in line with the general theoretical aspects of the photocatalytic reactions.
The results of kinetic studies show that the kinetic model for the photodegradation of 2-chlorophenol corresponds to the L-H model. The kinetic constant value obtained, k, was 0.22 mg L-1 min-1 and it is observed in Table 2 that the adsorption constant values depend on the mass of the photocatalyst and its morphological and crystallographic properties.
REFERENCES
1. Akpan, U.G. and B.H. Hameed, "Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review," Journals of Hazardous Materials, 170, 520-529 (2009).
2. Bekbolet, M., A.S. Suphandag and C.S. Uygmer, "An investigation of the photocatalytic efficiencies of TiO2 powders on the decolourisation of humic acids," J. Photochem. Photobiol A, 148, 121-128 (2002).
3. Farias, J., E.D. Albizzati and O.M. Alfano, "Kinetic study of the photo-Fenton degradation of formic acid. Combined effects of temperature and iron concentration," Catalysis Today, 144, 117-123 (2009).
4. Friedmann, D., C. Mendive and D. Bahnemann, "TiO2 for water treatment: Parameters affecting the kinetics and mechanisms of photocatalysis," Applied Catalysis B: Environmental, 99, 398-406 (2010).
5. Gondal, M.A., M.N. Sayeed and A. Alarfaj, "Activity comparison of Fe2O3, NiO, WO3, TiO2 semiconductor catalysts in phenol degradation by laser enhanced photo-catalytic process," Chemical Physics Letters, 445, 325-330 (2007).
6. Krijgsheld, K.R. and A. Van der Gen, "Assessment of the impact of the emission of certain organochlorine compounds on the aquatic environment. Part I. Monochlorophenols and 2,4-dichlorophenolds," Chemosphere, 15, 825-860 (1986).
7. Linsebigler, A.L., G. Lu and J.T. Yates Jr., "Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results," Chem. Rev., 95, 735-758 (1995).
8. Paasivirta, J., "Organochlorine compounds in the environment," Water Sci. Technol., 20, 119-129 (1988).
9. Peiró, A.A., J.A. Ayllón, J. Peral and X. Doménech, "TiO2-Phtocatalyzed degradation of phenol and ortho-substituted phenolic compounds," Applied Catalysis B: Environmental, 30, 359-373 (2001).
10. Roques, H., Chemical Water Treatment, VCH, Verlag Weinheim, Germany (1996).
11. Sobczynski, A., L. Duczmal and W. Zmudzinski, "Phenol destruction by photocatalysis on TiO2: an attempt to solve the reaction mechanism," Journal of molecular Catalysis A: Chemical, 213, 225-230 (2004).
12. Valente, J.P.S., P.M. Padilha and A.O. Florentino, "Studies on the adsorption and kinetics of photodegradation of a model compound for heterogeneous photocatalysis onto TiO2," Chemosphere, 64, 1128-1133 (2006).
13. Wang, K.R., Y.H. Hsieh, M.Y. Chou and C. Chang, "Photocatalytic degradation of 2-chloro and 2-nitrophenol by titanium dioxide suspensions in aqueous solution," Appl. Catal. B: Environ., 21, 1-8 (1999).
14. Wu, C.H., H.W. Chang and J.M., Chern, "Basic dye decomposition kinetics in a photocatalytic slurry reactor," Journal of Hazardous Materials, B137, 336-343 (2006).
15. Zhang, W., Y. Li, C. Wang and P. Wang, "Kinetics of heterogeneous photocatalytic degradation of rhodamine B by TiO2-coated activated carbon: Roles of TiO2 content and light intensity," Desalination, 266, 40-45 (2011).
Received: May 5, 2012
Accepted: January 9, 2012.
Recommended by Subject Editor: Pedro de Alcântara Pessôa.












