Servicios Personalizados
Articulo
Latin American applied research
versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.43 no.4 Bahía Blanca oct. 2013
Photo-induced curing of thymine-based bioinspired polymers. a chemometric analysis
S.A. Bortolato, A.L. Barbarini, R.M. Benitez, D.M. Martino and D.A. Estenoz
Instituto de Desarrollo Tecnológico para la Industria Química (INTEC) (UNL -CONICET), Güemes 3450, S3000GLN Santa Fe, Argentina. FAX: +45 342-4550944. destenoz@santafe-conicet.gov.ar
Abstract— The curing process of new materials based on styrene monomers functionalized with thymine and charged ionic groups was studied using UV-vis spectroscopy in combination with chemometric models. The effect of the copolymer molecular weight on the immobilization point was analyzed. The evolution of the curing process of the copolymer (4-vinylbenzyl) thymine (VBT) -vinylbenzyl triethyl ammonium chloride (VBA) involved three species, which absorb in the spectral region analyzed. The contributions of each species to the total signal at each irradiation time were determined, and the kinetic constant of the crosslinking reaction was estimated. The study allowed evaluating the consistency of the chemometric decomposition, obtaining a reasonable correlation between the frequency spectra and the time evolution calculated with the algorithm. The chemometric analysis showed to be a powerful tool to provide complementary information on photo-induced immobilization of VBT-VBA films, which is crucial for developing new environmentally benign materials and new energy-saving methods.
Keywords— Biopolymers; Thymine; Curing Kinetics; UV-vis Spectroscopy; Chemometrics.
I. INTRODUCTION
During the last decades, extensive research has been done to design environmentally benign synthetic polymers containing nucleic acid bases (Takemoto, 1976 and references therein; Inaki, 1992; Blackburn and Davies, 1966; Lamola and Mittal, 1966). In light of bioinspiration, polymers based on a new synthetic monomer, (4-vinylbenzyl) thymine (VBT), were designed to have the ability to photo-crosslink upon irradiation with the short-wavelength ultraviolet (UV) component of sunlight (Cheng et al., 1995; Grasshoff et al., 1995a; Grasshoff et al., 1995b). This process is bioinspired on the photo-dimerization of adjacent thymine pendant groups in DNA under UV irradiation (such as direct sunlight exposure), which disrupts the helical structure of DNA.
The VBT-based photo-polymers have potential applications in a variety of fields ranging from hair care products (Warner et al., 2004) to printed circuit boards and photo-imaging systems, (Grasshoff et al., 1997; Grasshoff et al., 1998; Lloyd-Kinstrand and Warner, 2003; Trakhtenberg et al., 2005) as well as controlled release systems for pharmaceutical and agricultural use (Saito et al., 2007; Saito and Warner, 2009; Kaur et al., 2011). Several studies related to the synthesis, the curing process, the properties and the applications of thymine-based VBT-vinylbenzyl triethyl ammonium chloride (VBA) polymers had been published (Lamola and Mittal, 1966; Cheng et al., 1995; Grasshoff et al., 1995a; Grasshoff et al., 1995b; Warner et al., 2004; Grasshoff et al., 1997; Grasshoff et al., 1998; Lloyd-Kinstrand and Warner, 2003; Trakhtenberg et al., 2005; Saito et al., 2007; Saito and Warner, 2009; Kaur et al., 2011; El-Hayek, 2004; Kiarie et al., 2005; Barbarini et al., 2010a; Trakhtenberg et al., 2007; Casis et al., 2007; Martino et al., 2008; Barbarini et al., 2010b; Warner et al., 2003; Whitfield et al., 2005). Saito et al. (2007, 2009) synthesized a series of micelle-forming block copolymer systems in aqueous solution and showed that guest materials could be encapsulated by hydrogen bonding with the attached thymine in the core. It was found that the enzyme DNA photolyase can "unzip" the crosslinking by catalyzing the reverse photo-crosslinking in thymine-containing styrene derivatives (Warner et al., 2003; Whitfield et al., 2005). Martino et al. (2008) reported a sensitization study on a family of water-soluble photo-polymers based on thymine, which clearly demonstrated that the presence of sensitizer molecules could promote the photo-dimerization of thymine-containing polymers in the visible range. In Casis et al. (2007) the synthesis of VBT-VBA polymers in solution at low temperatures (65°C) was theoretically and experimentally studied. A mathematical model for the free radical copolymerization of VBT and VBA was developed. The model allows predicting the global variables (conversion, composition) and the molecular structure (molecular weight distribution -MWD, chemical composition) of the copolymers along the reaction. Barbarini et al. (2010b) investigated the photo-induced UV curing kinetics of VBT-VBA copolymers from the UV absorption spectra measured along the process. The inmobilization point, related to the gel point, was determined following the evolution of the absorbance peaks of the copolymer solution after UV irradiation. The kinetics of the crosslinking reaction was estimated from the evolution of thymine concentration before the immobilization point considering additive contributions of VBT and VBA repetitive unit absorbances. A second order kinetics was observed and a significant effect of the film preparation on the degree of crosslinking was also detected. Apparent differences in the kinetics
constants were found for mixtures with different VBT: VBA ratio, due to the variations in the characteristics of the films. Experimental results suggested a higher content of remaining water in the (VBT)(VBA)4 films that produces a decrease in the vitreous transition temperature (Tg) and therefore a higher mobility of the polymer chains. In addition, the presence of other compounds that might be generated in the reaction medium while irradiating was not considered. These species could absorb in the same spectral region and, in this case, the absorbance of the copolymer solution would not be exactly proportional to the concentration of VBT.
UV-vis spectroscopy allows the study of the curing process in real time, without the need for sample pre-treatments. However, the lack of signal specificity in the obtained spectra prevents the correct characterization of the species involved in the curing process. These spectra are typically used in a univariated way, analyzing the changes produced in a characteristic band and relating them to the overall progress of the reaction. While this type of analysis enables obtaining adequate information to assess the degree of total conversion of reactants to products, it does not provide information about the number of steps involved in the process, the concentration of each chemical species along the time and their corresponding wavelength spectra.
In order to obtain more information about a complex system, chemometric methods are meaningful tools because they allow a multivariate analysis of spectral data. In this way, the required information on compounds of analytical relevance can be extracted in a fast and reliable way from the mathematical treatment of the data, without the need of a physical prior separation (De Juan and Tauler, 2003). In the last two decades, these tools have been widely used to solve problems of broad physical-chemical nature (Olivieri, 2008). Specifically, a very convenient method applied to spectroscopic data monitoring chemical reactions processes, i.e. curing reactions, is Multivariate Curve Resolution Algorithm assisted by alternating least squares (MCR-ALS) (Jaumot et al., 2005). Garrido et al. (2008) performed a comprehensive review of the multiple posibilities of this algorithm to solve different chemical reactions. Larrechi and Rius (2004) showed the use of MCR-ALS to study the curing reactions of epoxy resins, obtaining the concentration profiles as a function of time for the chemical species involved in the reaction and their corresponding spectra. On the other hand, Spegazzini et al. (2009) use MCR-ALS to analyze FTIR data and obtained representative spectra of the compounds that participate in the curing reaction between diglycidyl ether of bisphenol A and γ-valerolactone. From the best of our knowledge, the use of MCR-ALS applied to UV-Vis spectroscopical data to study photo-induced curing reactions has not being reported.
The condition required to apply MCR-ALS is that the data have a bilinear structure. This means that the experimental data matrix (matrix of intensities of response), commonly called D, can be expressed as the product of a concentration matrix by another matrix containing the raw signal of the existing species, G and S respectively (De Juan and Tauler, 2003). This condition is fulfilled by most spectroscopic techniques if the Beer-Lambert law is satisfied. Thus, by decomposing a data matrix generated during the monitoring of a crosslinking reaction, it is possible to know the evolution of each species that occur in the process and their corresponding pure spectra. One advantage is that this model allows obtaining the information without the need to understand the reaction mechanism or to establish a specific kinetic model previously (De Juan et al., 2000).
In this work, the main goal is to apply the MCR-ALS chemometric analysis of UV-Vis spectral data in order to determine the photo-induced crosslinking kinetics of VBT-VBA copolymers. To this effect, copolymer mixtures of different VBT-VBA compo-sition were irradiated at 254 nm for different times (Scheme 1). Thereafter, the absorbance spectra in the region of 240-310 nm were recorded and this information was used by chemometric algorithm to estimate the kinetic constant of the curing process.
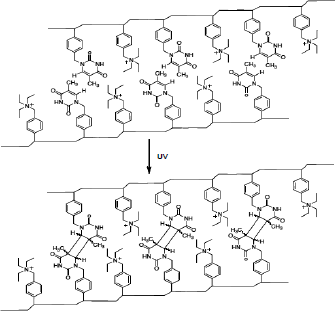
Scheme 1. Photo-dimerization of VBT-VBA copolymers under UV irradiation.
II. METHODS
As it was mentioned in the introduction, in a previous work on the photo-induced UV curing kinetics of VBT-VBA copolymer systems(Barbarini et al., 2010b) apparent differences were observed in the kinetics constants of mixtures with different VBT:VBA ratio, suggesting a significant effect on the degree of cross-linking due to the film preparation. Consequently, a new set of polymerization reactions along with complete product characterization were carried out following the reported recipes (Barbarini et al., 2010b), and new curing reactions were completed in order to take in consideration and avoid possible sources of error.
A. Copolymer Synthesis and Characterization
All the reagents were purchased from Sigma-Aldrich and used as received unless otherwise specified. VBT was synthesized from thymine and vinylbenzyl chloride, while VBA was synthesized from vinylbenzyl chloride and triethylamine, as described previously (Cheng et al., 1995). The UV-vis absorption spectra of pure monomers in aqueous solution (0.02 mg VBT/mL and 0.3 mg VBA /mL) were measured using a UV-vis Spectrometer PerkinElmer Lambda 20.
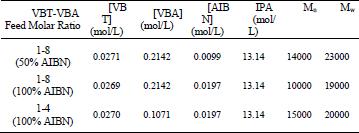
In order to produce water-soluble polymers, VBT was copolymerized with the cationic monomer VBA in a free radical process. VBT-VBA copolymerization reactions were carried out using different molar ratios of the monomers [(VBT)(VBA)m, m=4,8] and different concentrations of chemical initiator [AIBN] (see Table 1). All reactions were carried to complete conversion. Table 1 presents the recipes and characterization results of the final products.
Table 1.Recipes and Characterization Results for the Final Products.
To verify the absence of unreacted monomers, the precipitated polymer was analyzed by H NMR spectroscopy (Bruker 300 MHz) and the typical vinyl group signal at chemical shifts between 5 and 6 ppm was not observed in the spectra.
Gel permeation chromatography (GPC) was used for the determination of the copolymer MWD, the weight and number averages molecular weights (Mw and Mn, respectively). An Agilent 1100 chromatograph was fitted with a set of TSK PW 2500 and 4000 columns and equipped with a differential refractometer. The carrier solvent was a 70/30 buffer mixture of water/methanol containing sodium acetate (0.5 M) and acetic acid (0.5 M) with a flow rate of 1.0 ml/min. The calibration curves were obtained from polyethylene oxide standards dissolved in the same buffer. The data treatment was carried out with Agilent GPC data analysis software. In Table 1, the Mn and Mw of the copolymer at the end of the reaction are shown.
B. Copolymer Curing: coating preparation, film irradiation and development
Aqueous solutions 10% w/w of (VBT)(VBA)m polymers, with m=4, 8 where prepared and homogenized by sonication at 60°C. Hydrophilic polyethylenterephthalate film (PET-X4C1, Dupont) was used as substrate without previous treatment. A known amount of aqueous polymer solution was distributed homogeneously using a #03 wire-round milled coating rod (R.D. Specialties Inc., Webster NY), which resulted in coatings with wet thickness of 6.8 μm (Mac Leod, 1991). The films were dried at room temperature for one hour and in the oven at 80°C for another hour. The PET film was cut in samples of equal size and irradiated for different times with a short wavelength UV hand lamp (Spectroline UL, Model ENF 260c) at 254 nm and intensity of 1.3x10-3 W/cm2. Light intensity was measured with a photo-detector (International Light INC. IL 1700). After irradiation, each sample was rinsed in 1 ml of distilled water for 60 seconds to remove the uncrosslinked polymer from the PET surface (Barbarini et al., 2010b).
Finally, the crosslinking was monitored by UV-vis spectroscopy as a function of irradiation time (equivalent to the amount of energy delivered). Spectra for each washing solution were recorded between 240 and 310 nm every 1 nm (71 wavelengths) for the following irradiation times: 0, 5, 8, 10, 12, 15 and 18 seconds (7 irradiation times) for the copolymer (VBT)(VBA)4, and 0, 5, 10, 20, 30, 40, 43, 45, 48 and 50 seconds (10 irradiation times) for (VBT)(VBA)8. This procedure was carried out by triplicates and the average values are reported. The spectra were saved in ASCII format and transferred to a PC microprocessor Athlon X2 Dual-Core QL-60 (1.90 GHz) for further manipulation.
C. Chemometric Analysis
MCR-ALS was used to mathematically decompose the total signal of each UV absorption spectra into the contribution of individual species, in order to quantify the content of each component in these spectra (De Juan and Tauler, 2003).
The bilinear decomposition for the matrix D is performed using the following equation:
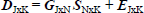 | (1) |
where the J rows of D contain the spectra measured for different samples at each time K. The columns of G contain the temporal profiles of the N species involved in all the experiments and the columns of S represent the spectra related to these species. Finally E is the matrix of the residuals not adjusted by the bilinear decomposition. In the analyzed cases, the matrix D has the following dimensions: for (VBT)(VBA)4, 71 [wavelengths] x 7 [irradiation times], and for (VBT)(VBA)8, 71 x 10.
The decomposition of D is done through an iterative minimization procedure by alternating least squares of the Frobenius norm of E. The minimization is initiated by providing estimated spectra for the different spectral components that are used to estimate G as follows,
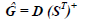 | (2) |
where the symbol "^" indicates that it is a matrix estimated from Eq. (1), "T" means the transposed matrix, and the superscript "+" the generalized inverse. From Ĝ, and the original data matrix D, the spectral matrix S is recalculated by the least squares:
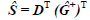 | (3) |
E is obtained from Eq. (1) using D and the estimated matrices Ĝ and Ŝ. These steps are repeated until the convergence is reached. The algorithm is fitted with initial restrictions to achieve greater convergence throughout the process. It is necessary that the system is non-negative in two dimensions, both in the irradiation time direction and in the wavelength direction.
The MCR-ALS algorithm requires the exact number of factors responsible for the analytical signal, and it is preferable to initialize the system with the profiles of the components as close as possible to the final result. The number of factors is estimated using principal com-ponent analysis based on singular value decomposition of the matrix D (Maeder and Zilian, 1988).
Finally, the spectra of the species can be obtained from the analysis of the so called "pure" spectra, following the method SIMPLISMA (SIMPLe Interactive Self-modeling Mixture Analysis), an algorithm of multivariate resolution that extracts pure spectra from mixtures of variable composition (Windig and Guilment, 1991). After the MCR-ALS decomposition of the D matrix is obtained, the information contained in G can be used to estimate the individual contributions of each species to the absorbance measured at each time. On the other hand, S contains the spectra of the different components, which are of great interest to understand how the curing process evolves.
The routines used for MCR-ALS were performed in MATLAB 7.0 (2007) and are freely available on the Internet (http://www.ub.es/gesq/mcr/mcr.htm). Chemo-metric analysis processing took less than five minutes for each matrix data.
III. RESULTS AND DISCUSSION
To study the kinetics of the crosslinking process, the evolution of the concentration of unreacted thymine present in the washing solution was followed at the beginning of the curing stage. UV-vis spectroscopy gives information about the conjugation extent in the copolymer, which decrease when the conjugated C=C double bond of the thymine splits to form the dimer after irradiation at 254 nm. Figure 1 shows the evolution of the UV spectra for the washing solutions of copolymers (VBT)(VBA)4 and (VBT)(VBA)8 obtained with different AIBN initiator concentrations (see Table 1). The absorption peak at 270 nm which is correlated with the amount of thymine units present in solution, is reduced in accordance with the increase of photo-dimerization degree.
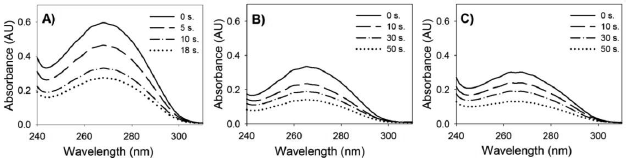
Fig. 1.UV-vis absorption spectra of unreacted polymer in the washing solution as function of wavelength for some irradiation time points: A) (VBT)(VBA)4100% AIBN, B) (VBT)(VBA)8100% AIBN, and C) (VBT)(VBA)850% AIBN.
Figure 2 shows the decay of the maximum absorption peak at 270 nm as function of the irradiation time obtained from the absortion spectra like in Fig. 1. It can be observed a sudden decrease of the absorbance peak. This position was related to the immobilization point where the crosslinked polymer chains that remain at-tached to the PET substrate reduce the amount of un-crosslinked thymine moeities in the washing solution (Barbarini et al., 2010b). From Fig. 2, this point occurs after approximately 15 seconds of irradiation for (VBT) (VBA)4, and after 40 seconds for (VBT)(VBA)8 with 100% AIBN. The delay in the immobilization time is due to the lower thymine concentration in the polymer. The effect of the prepolymer molecular weight on the immobilization point is clearly observed (Fig. 2b). As expected, the higher the molecular weight [(VBT) (VBA)8 with 50% AIBN], the shorter the immobiliza-tion time (approximately 30 seconds).
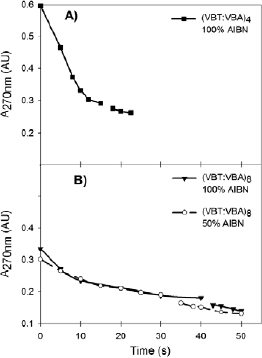
Fig. 2. Time evolution of the absorbance peak at 270 nm corresponding to the amount of thymine units in solution, for copolymers: A) (VBT)(VBA)4100% AIBN, and B) (VBT) (VBA)8, for two initiator concentration (50 and 100% AIBN). Solid lines are used as guides for eyes only.
The kinetics of the crosslinking reaction can be estimated from the thymine concentration during the curing process before the immobilization point. However, the concentration of thymine can not be obtained directly from the absorption peak at 270 nm, because the absorbance measured at that wavelength is composed of contributions from at least three species: the conjugated double bonds of VBT monomer, the conjugated double bonds of VBA and the conjugated double bonds of a new specie called "dimer", coming up as a result of the curing evolution. Figure 3 shows the UV-vis absorption spectra of aqueous solution of pure VBT and VBA monomers.
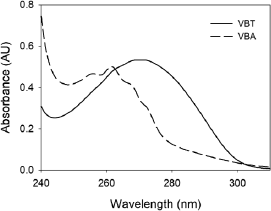
Fig. 3. UV-vis absorption spectrum as function of wavelength for aqueous solution of VBA at 0.3 mg/ml (dashed line) and VBT at 0.02 mg/ml (solid line).
To determine the concentration of thymine, MCR-ALS algorithm is used. In this sense, it is expected that the signal of VBT monomer decreases as the curing reaction proceeds, that the VBA monomer signal remains roughly constant as the corresponding double bonds are not affected by irradiation at 254 nm, and that the new signal corresponding to the thymine dimer increases proportionally to the decrease in VBT signal.
Figure 4 shows the different data matrices for the VBT-VBA copolymers analyzed both in the three-dimensional representation and the contour graph. These arrays were obtained from the generating of the D matrix as it was described in the Experimental Section and a later processing by MCR-ALS.
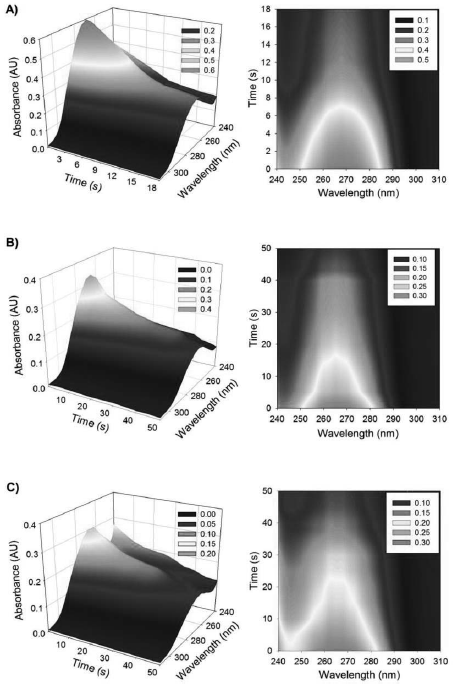
Fig. 4. Three-dimensional representation and contour map of the experimental data for: A) (VBT)(VBA)4 with 100% AIBN, B) (VBT)(VBA)8 with 100% AIBN and C) (VBT)(VBA)8 with 50% AIBN.
In Fig. 4 it can be observed a clear decrease of the signal intensity when the irradiation time increases, which is consistent with what was shown in Fig. 2.
The total absorbance results are lower in the case of (VBT)(VBA)8 copolymers, due to less amount of conjugated double bonds of thymine in VBT. The contour graphs show a very noticeable spectral shifts of the maximum peak towards lower wavelengths which can be explained by an increase of the amount of VBA monomer in the samples having a maximum peak at 260 nm (see Fig. 3). Due to these experimental results, a supplementary data analysis based on chemometrics approach is adopted.
Figure 5 shows the kinetic and spectral information obtained by applying the chemometric algorithm (Eqs. 1-3) to the experimental data presented in Fig. 4. For the copolymers obtained with all monomer ratios and AIBN initiator concentrations, the absorption spectra calculated for the VBA and VBT monomers (Fig. 5A-5C) are similar to the UV spectra recorded for each pure monomer (Fig. 3), while the spectrum of the new species can be correlated reasonably well with the product of the crosslinking reaction (Lamola and Eisinger, 1968). This information is further confirmed by the kinetics profiles that are calculated with the algorithm (Fig. 5D-5F). For the VBT signal, the concentration decreases with increasing irradiation time, while virtually the VBA signal remains constant over time. On the other hand, it can be noticed that there is an increase of the signal corresponding to the new specie, which is consistent with the decrease in VBT signal. Additionally, the contribution of the different species to the total measured absorbance for each irradiation time can be determined from the time profiles.
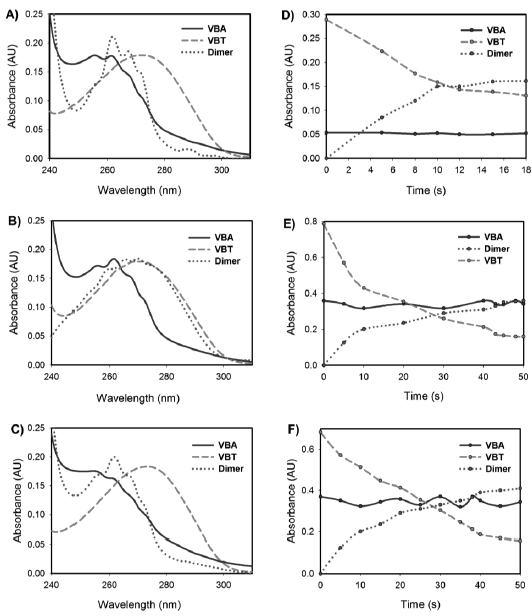
Fig. 5. Spectral profiles and absorbance evolution along the irradiation time resolved by MCR-ALS for: A,D) (VBT)(VBA)4 with 100% AIBN; B,E) (VBT)(VBA)8 with 100% AIBN; and C,F) (VBT)(VBA)8 with 50% AIBN. Dashed red lines for VBT, solid blue lines for VBA and dotted green lines for Dimer
Finally, the evolution of pure thymine concentration obtained by this method was used to characterize the kinetics of the curing process at the beginning of the crosslinking, before the gel point. The time evolution of the inverse of thymine concentrations (1/[Ty]) for (VBT)(VBA)4 and (VBT)(VBA)8 copolymers with different initiator concentrations are represented in Fig. 6. In all cases, a linear evolution was observed indicating a
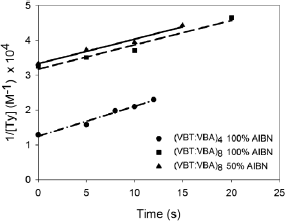
Fig. 6. Inverse of thymine concentration as function of irradiation times for copolymers (●) (VBT)(VBA)4, and (VBT)(VBA)8 with different AIBN initiator concentrations (▲) 50% AIBN and (■) 100% AIBN. Dash-dotted, solid and dashed lines represent the linear regression fittings, respectively.
second order kinetics for the crosslinking process with respect to thymine concentration. From the linear least square fittings (values of R2 higher than 0.99 in all cases), the rate constant of the crosslinking reaction at 254 nm was determined, with an average value of 1030 L/ mol s-1. The single value obtained for the slope of the evolutions confirms the identical crosslinking rate constant for all monomer ratios and AIBN initiator concentrations. The finding of only one rate constant value is in contrast with previous results where different crosslinking rates were found (Barbarini et al., 2010b), reinforcing the fact that the film characteristics are crucial in the curing process. Also, as it was expected the intercept values coincides with the inverse of the initial thymine concentrations: 0.8x10-4 M and 0.3x10-4 M for (VBT)(VBA)4 and (VBT)(VBA)8 copolymers, respectively.
IV. CONCLUSIONS
The curing process of new materials based on styrene monomers functionalized with thymine and charged ionic groups were studied using UV-vis spectroscopy in combination with a MCR-ALS chemometric model. The effect of the molecular weight of the copolymer on the "immobilization point" was analyzed. The deter-mination of this point is relevant to establish the curing conditions necessary to produce a crosslinked polymer.
A more accurate characterization of the evolution of the crosslinking process was accomplished, obtaining a good estimation of the kinetics rate constant of the process. To that effect, it was determined that the curing process of the copolymer VBT-VBA involved three species which absorb in the spectral region analyzed. Furthermore, it was possible to determine the contribution of each species to the total signal at each irradiation time, which enabled to estimate the kinetic constant of the process only as a function of VBT, which is the most important analytical compound. Additionally, the study allowed evaluating the consistency of the chemometric decomposition obtaining a reasonable correlation between the frequency spectra and the time evolutions obtained with the algorithm. In summary, it was possible to use the developed chemometric tool to provide complementary information on photo-induced immobilization of VBT-VBA films that are crucial for developing new environ-mentally benign materials and new energy-saving methods.
Kinetics results can be used in statistical mathemati-cal models that simulate the crosslinking process in function of the prepolymer molecular structure and the curing conditions. The final goal is to optimize the synthesis and curing processes to obtain materials with pre-specified properties and quality.
ACKNOWLEDGEMENTS
DMM and DAE are members of the Research Council of CONICET. Authors would like to thank Universidad Nacional del Litoral (CAI+D Tipo II PI 11-57), CONICET (PIP 112-200801-01079) and Fundación Nuevo Banco de Santa Fe (FNBSF) for the financial support.
REFERENCES
1. Barbarini A., D. Reyna and D.M. Martino, "Effect of light intensity and film thickness on the photo-crosslinking of Poly(Vinyl Benzyl Thymine-co-Triethyl Ammonium Chloride)," Green Chemistry Letters and Reviews, 3, 231-237 (2010a).
2. Barbarini, A., D. Martino and D. Estenoz, "Synthesis, Curing and Characterization of Bioinspired Polymers based on Vinyl Benzyl Thymine and Triethyl Ammonium Chloride," Macromol. React. Eng., 4, 453-460 (2010b).
3. Blackburn, G.M. and R. J. H. Davies, "The structure of thymine photo-dimer," J. Chem. Soc. C., 23, 2239-2244 (1966).
4. Casis, N., C.V. Luciani, J. Vich Berlanga, D.A. Estenoz, D.M. Martino and G.R. Meira, "Synthesis of "bioinspired" copolymers: experimental and theoretical investigation on poly(vinyl benzyl thymine-co-triethyl ammonium chloride)," Green Chemistry Letters and Reviews, 1, 62-75 (2007).
5. Cheng, C.M., M.I. Egbe, J.M. Grasshoff, D.J. Guarrera, R.P. Pai, J.C. Warner and L.D. Taylor, "Synthesis of 1-(vinylbenzyl)thymine, a novel, versatile multi-functional monomer," J. Polym. Sci., Part A: Polym. Chem., 33, 2515-2519 (1995).
6. De Juan, A., E. Casassas and R. Tauler, Encyclopedia of Analytical Chemistry; Ed. R.A. Meyers; John Wiley & Sons, Ltd. Chichester, England (2000).
7. De Juan, A., and R. Tauler, "Chemometrics applied to unravel multicomponent processes and mixtures-Revisiting latest trends in multivariate resolution," Anal. Chim. Acta, 500, 195-210 (2003).
8. El-Hayek, R., Bacteriostatic Polymeric Film Immobilization, MS Thesis, UMASS, Boston (2004).
9. Garrido, M., F.X. Rius and M.S. Larrechi, "Multivariate Curve Resolution-Alternating Least Squares (MCR-ALS) applied to spectroscopic data from monitoring chemical reactions processes," Anal. Bioanal. Chem., 390, 2059-2066 (2008).
10. Grasshoff, J.M., L.D. Taylor and J.C. Warner, U.S. Patent 5,395,731, March 7 (1995a).
11. Grasshoff, J.M., L.D. Taylor and J.C. Warner, U.S. Patent 5,455,349, October 3 (1995b).
12. Grasshoff, J.M., L.D. Taylor and J.C. Warner, U.S. Patent 5,616,451, April 1 (1997).
13. Grasshoff, J.M., L.D. Taylor and J.C. Warner, U.S. Patent 5,708,106, January 13 (1998).
14. Inaki, Y., "Thymine Polymers as High Resolution Photoresists and Reversible Photo-recording Materials," Polym. News, 17, 367-371 (1992).
15. Jaumot, J., R. Gargallo, A. de Juan and R. Tauler, "A graphical user-friendly interface fo r MCR -ALS: a new tool for multivariate curve resolution in MATLAB," Chemom. Intell. Lab. Syst., 76, 101-110 (2005).
16. Kaur, G., S.L.Y. Chang, T.D.M. Bell, M.T.W. Hearn and K. Saito, "Bioinspired core-crosslinked micelles from thymine-functionalized amphiphilic block copolymers: Hydrogen bonding and photo-crosslinking study," J. Polym. Sci., Part A: Polym. Chem,. 49, 4121-4128 (2011).
18. Kiarie, C., J. Bianchini, S. Trakhtenberg and J.C. Warner, "Methylene Blue Adsorption on Thymine. Based Polyvinylphenylsulfonate Films," J. Macromol. Sci. Part A Pure and Appl.Chem., 42, 1489-1496 (2005).
19. Lamola A.A. and J. P. Mittal, "Solution Photochemistry of Thymine and Uracil," Science, 154, 1560-1561 (1966).
20. Lamola A.A. and J. Eisinger, "On the mechanism of thymine photodimerization," Proc Nac. Acad. Sci: Chemistry, 59, 46-51 (1968).
21. Larrechi, M.S., and F.X. Rius, "Spectra and concentration profiles throughout the reaction of curing epoxy resins from near-infrared spectroscopy and multivariate curve resolution methods," Appl. Spectrosc., 58, 47-53 (2004).
22. Lloyd-Kindstrand, L. and J.C. Warner, Biopolymers; Eds. S. Matsumura, A. Steinbüchel, Wiley: Weinheim, Germany, 9, 165-174 (2003).
23. MacLeod, D.M., Coating Technology Handbook; Ed. D. Satas, Marcel Dekker: New York (1991).
24. Maeder, M. and A. Zilian, "Evolving factor analysis, a new multivariate technique in chromatography," Chemom. Intell. Lab. Syst., 3, 205-213 (1988).
25. Martino, D.M., D. Reyna, D. Estenoz, S. Trakhtenberg and J.C. Warner, "Photosensitization of Bioinspired Thymine Containing Polymers," J. Phys. Chem. A, 112, 4786-4792 (2008).
26. MATLAB 7.0, The Mathworks, Natick, Massachussets, USA (2007).
27. Olivieri, A.C., " Analytical advantages of multivariate data processing. One, two, three, infinity?," Anal. Chem., 80, 5713-5720 (2008).
28. Saito, K., L.R Ingalls, J. Lee and J.C. Warner, "Core-bound polymeric micellar system based on photocrosslinking of thymine," Polym. Commun. 2503-2505 (2007).
29. Saito, K., and J.C. Warner, "Core-shell thymine containing polymeric micelle system: study of controlled release of riboflavin," Green Chemistry Letters and Reviews, 2, 71-76, (2009).
30. Spegazzini, N., I. Ruisánchez and M.S. Larrechi, "MCR-ALS for sequential estimation of FTIR-ATR spectra to resolve a curing process using global phase angle convergence criterion," Anal. Chim. Acta, 642, 155-162 (2009).
31. Takemoto, K., "Functional monomers and polymers containing nucleic acid bases," J. Polym. Sci. Polym. Symp., 55, 105-125 (1976).
32. Trakhtenberg, S., Y. Hangun-Balkir, J.C. Warner, F.F. Bruno, J. Kumar, R. Nagarajan and L.A. Samuelson, "Photo-Cross-Linked Immobilization of Polyelectrolytes for Enzymatic Construction of Conductive Nanocomposites," J. Am. Chem. Soc., 127, 9100-9104 (2005).
33. Trakhtenberg, S., R. Kumar, J. Bianchini, S. Thor, D. Martino and J.C. Warner, "Influence of pH and salt on the photocrosslinking in polyelectrolyte thymine-containing films," J. Macromol. Sci. Part A Pure and Appl.Chem., 44, 1311-1318 (2007).
34. Warner, J.C., A. Morelli and M.C. Ku, U.S. 4 pp US 2003224497 (2003).
35. Warner, J.C., A.S. Cannon, J. Raudys and A. Undurti, US Patent 02,06,368, October 21 (2004).
36. Whitfield, J., A. Morelli and J.C. Warner, "Enzymatic Reversal of Polymeric Thymine Photocrosslinking with E. coli DNA Photolyase," J. Macromol. Sci. Part A Pure and Appl. Chem., 42, 1541-1546 (2005).
37. Windig, W. and J. Guilment, "Interactive Self-Modeling Mixture Analysis," Anal. Chem., 63, 1425-1432 (1991).
Received: August 27, 2012
Accepted: October 18, 2012
Recommended by Subject Editor: Mariano Martín Martín.