Servicios Personalizados
Articulo
Latin American applied research
versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.44 no.1 Bahía Blanca ene. 2014
Use of a free radical scavenging method on extracts obtained by molecular distillation from oregano essential oil
A.V. Borgarello†, G.N. Mezza†, A.T.Soltermann‡ and M.C. Pramparo†
† Dep. Tec. Qca, Fac. de Ing., Univ. Nac. de Río Cuarto, Ruta 6 km. 301, (5800) Río Cuarto, Argentina.
aborgarello@ing.unrc.edu.ar; mpramparo@ing.unrc.edu.ar
‡ Applied Chemistry Department, University of the Basque Country (UPV-EHU), San Sebastián, 20080, Spain
Abstract — There is evidence that oregano essential oil has a significant antioxidant effect on the process of lipid oxidation. In order to obtain fractions enriched in antioxidant properties, oregano essential oil samples were processed by molecular distillation. Molecular distillation experiments were done in two groups of tests, using an evaporating temperature between 22 and 30 °C. Pressure was varied between 0.7 and 53 mbar.
Antioxidant activity, specifically radical scavenging capacity, was analyzed in the essential oil and the distillates and residues obtained by a free radical scavenging method, using DPPH* (2,2-diphenyl-1-picrylhydrazyl). The results obtained allowed to conclude that the free radical scavenging capacity was increased in the residue fractions obtained by molecular distillation. These fractions were concentrated in thymol. The IC50 values of the residues were similar to the synthetic antioxidant BHT, suggesting that fractions concentrated in oxygen derivatives obtained by molecular distillation could be used in the food industry to retard lipid degradation.
Keywords — Essential Oils; Oregano; Molecular Distillation; Thymol; Antioxidants.
I. INTRODUCTION
The synthetic antioxidants more widely used in the food industry, butyl hydroxy toluene BHT, hydroxybutylanisole BHA, tertiary butylhydro-quinone TBHQ and propyl galate, are suspected to cause or promote negative effects on health. For this reason, there is a growing interest in the study of less toxic natural antioxidants. Potential sources of such antioxidants are herbs and spices because their essential oils are usually rich in phytochemicals with antiperoxidative characteristics and free radical scavenging properties (Bozin et al., 2006; Hazzin et al., 2006). These antioxidant properties are due to the synthesis and accumulation of secondary metabolites that occur in the plant organs in response to stimulation and environmental conditions. Among these metabolites there are substances with free radical scavenging properties, such as phenolic compounds, carotenoids, vitamins and nitrogen compounds (Burt, 2004; Zheng and Wang, 2001). Recently, several vegetal sources have been studied, finding herbs and spices whose essential oils and extracts exhibit antioxidant properties and are effective in the retardation of lipid oxidative degradation, preserving the quality and nutritional value of food (Bozin et al., 2006; Dambolena et al., 2010; Loizzo et al., 2009; Bakkali et al., 2008; Wojdylo et al., 2007; Shan et al., 2005).
Essential oils are complex mixtures which contain several components (20-60) at different concentrations. They are characterized by having few major components that are present at fairly high concentrations (20-70%) compared to others which exist as traces. Generally, these mayor constituents determine the biological properties of essential oils. The compounds present in essential oils include two groups of different biosynthetic origin. The main group is composed of terpenes and terpenoids and the other is formed by aliphatic compounds, all of them characterized by having low molecular weight (Bakkali et al., 2008).
Several studies on antioxidant activities of herbal essential oils have reported that oregano essential oil has significant antioxidant effect on the process of lard oxidation (Wojdylo et al., 2007; Milos et al., 2000; Tsimogiannis et al., 2006).
Antioxidant properties of plant extracts and pure compounds can be measured by various analytical techniques based on different characteristics of the antioxidant activity, such as free radical scavenging capacity, inhibition of the lipid peroxidation, and others (Bozin et al., 2006). Among the methods for testing the free radical scavenging capacity, the most studied have been ABTS+ and DPPH* (Wojdylo et al., 2007). Evaluation of antioxidant capacity and reproducibility of these methods has been good in certain test conditions depending on the application under study.
The DPPH* molecule is characterized as a stable free radical of violet color with an absorption band centered around 515 nm in alcoholic solution. When reacting with a substance that can donate a hydrogen atom (antioxidant), it is reduced, with the consequent loss of purple color.
It is possible to obtain products with high antioxidant properties by concentrating the oregano essential oil. One of the promising techniques for obtaining concentrates from essential oils is molecular distillation (Plazas Tovar et al., 2010; Rossi et al., 2011). There is little information in the literature on the application of this technique to essential oils. Molecular distillation is characterized by a short exposure of the distillate liquid to elevated temperatures, high vacuum in the distillation space, and short distance between the evaporation and condensation surfaces. The short residence time of the liquid in the evaporator, in the order of a few seconds to tens of seconds, is guaranteed by the distribution of the liquid in the form of a thin film of homogeneous thickness. Under these conditions, i.e., short residence times and low temperatures, the distillation of thermosensitive materials is accompanied by only negligible thermal decomposition and proceeds at technologically acceptable rates (Wang et al., 2009).
The aim of this work was to obtain, by molecular distillation, fractions of oregano essential oil enriched in antioxidant properties, and to evaluate those properties by a free radical scavenging method using the stable radical DPPH* as a reagent.
II. METHODS
A. Plant material and isolation of the essential oil
Oregano (Origanum vulgare L., ssp hirtum) was collected in the central area of Argentina. The plant material consisted of leaves and stalks. Air-drying of oregano was performed in a shady place at room temperature for 10 days. The plant material was used for the isolation of the essential oil, immediately after drying.
The oregano essential oil was isolated by 2-h steam stripping of 150-300 grams of the dried plant material using a steam distillation apparatus. The body of the distillation apparatus is made of pyrex glass and it is provided with an electrical resistance and liquid level control. It allows the use of one or two baskets of the same volume, which were filled with metallic Rasching rings to improve the contact between the two phases involved.
Ethanol was of HPLC purity and it was purchased from Reagents S.A. The standard compounds were purchased from Sigma-Aldrich and Fluka analytical. DPPH* was obtained from Sigma-Aldrich.
B. Essential oil concentration
Molecular distillation was performed in a KDL4 molecular distillator, manufactured by UIC Gmbh (Germany). It consists of a falling film evaporator (area= 4 dm2) and an internal condenser (area = 2 dm2). A scheme of the molecular distillation apparatus is shown in Fig. 1.
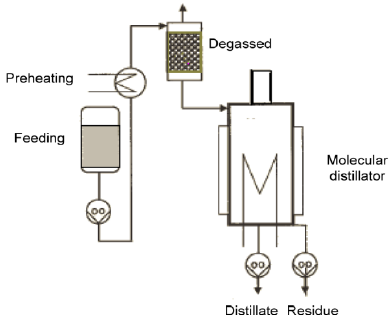
Fig. 1. One stage molecular distillator scheme
Two groups of experiments were carried out, with two different samples of oregano essential oil. In the first groups of experiments, the variation of the distillate quantity and the antioxidant activity were observed by the modification of the operation pressure. According to the results of the first group of experiments, the second group was design as a three stage molecular distillation in order to search a relation between composition of the different extracts and the antioxidant capacity. All the tests were repeated. Statistical analysis was performed by comparing means using Least Significant Difference (LSD) (Montgomery and Runger, 1996).
C. Determination of radical scavenging ability
The antioxidant activity of oregano essential oil and its fractions was measured in terms of their radical scavenging ability (Brand-Williams et al., 1995), using a technique with the stable radical DPPH* as reagent.
Ethanolic solutions with different concentrations (0.001 to 50 mg.ml-1) of essential oil, its fractions and commercial antioxidants were prepared. 2 ml of each sample were mixed with 2 ml of a DPPH* solution (90 μM). Absorbance of the resulting solutions and the control sample (with same chemicals, except sample) was measured after 180 min (for both the essential oil and its fractions) and after 30 min (for commercial antioxidants). The absorbance was recorded at 515 nm by a Metrolab 330 spectrophotometer. Ethanol was used to zero the spectrophotometer.
To determine the reaction time for essential oil and its fractions, a 10 mg.ml-1 ethanolic solution of the essential oil was prepared and mixed with 2 ml of 90 μM DPPH* solution. According to the technique described before, absorbance measurements were taken every 15 s to follow the absorbance change in time. A graph plotting absorbance value against time was constructed (Fig. 2). The reaction time (180 min) was set as the time where the absorbance values remained almost constant.
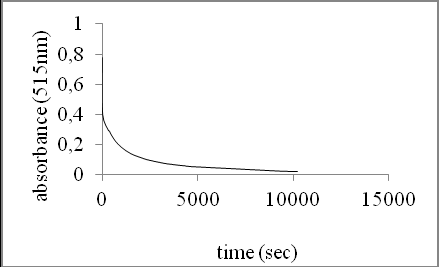
Fig. 2. Absorbance value against reaction time for a 10 mg. ml-1 solution
The percentage inhibition of the DPPH* free radical by the presence of antioxidants in the samples was calculated according to:
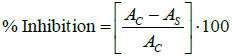
where AC is the absorbance of the control sample at t=180 min and AS is the absorbance of the antioxidant sample at t=180 min.
Sample concentration providing a 50% inhibition of the DPPH* radical was calculated from the graph plotting inhibition percentage against sample concentration. This concentration is called IC50 and it was the parameter used to compare antioxidant activity of the samples due to their radical scavenging ability.
D. Composition determination by GC
The quantification of the mayor components of the oregano essential oil and the fractions obtained by molecular distillation was made by gas chromatography, using a Hewlett Packard HP 6890 gas chromatograph, with an HP Innowax column, equipped with a flame ionization detector. Nitrogen was used as carrier gas with a flow rate of 0.7 ml.min-1. Samples were diluted with dichloromethane and the injection volume was 1 μL. Column temperature was gradually increased from 60 °C to 250 °C at 2°C.min-1, and finally held isothermal for 10 min. The injection port temperature was 250 °C and the detector temperature was 350 °C.
The main components were identified by comparing their retention time with standard compounds. Determination of the percentage composition of samples was based on peak area normalization without using correction factors.
E. Results and discussion
The dried oregano leaves and stalks yielded 1.5 % w/w of essential oil. In Table 1 the results of four test of molecular distillation are shown. These tests were carried out at 28±2°C (evaporating temperature) and 1.2±0.1 ml.min-1 of feed flowrate. Operation pressure and percentages of residue and distillate are listed. Variations in the operating pressure modified the evaporation efficiency, obtaining higher percentage of distillate at lower pressure. Also the IC50 values for distillate and residue are shown. The fractions of residue obtained by molecular distillation showed an increment of antioxidant capacity, while the distillates showed a decrease in that antioxidant capacity.
Table 1. Mass percentages of residue and distillate obtained by molecular distillation. Evaporating temperature: 28±2°C. Feed flowrate: 1,2±0.1 ml.min-1. IC50essential oil:1.2 mg.ml-1
Figure 3 shows that the parameter IC50 of these fractions is dependent of the percentages of distillate and residue obtained in each essay.
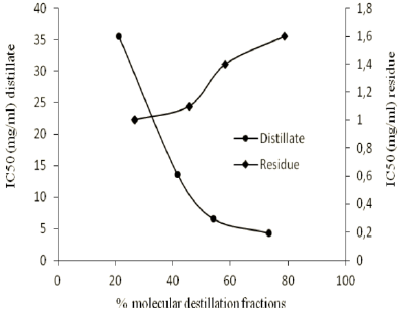
Fig. 3. Variation of IC50 on percentage of fraction obtained by molecular distillation.
Multistage distillation was performed using a flow rate of 1±0.1 ml.min-1 and an evaporating temperature of 24±2 °C. Pressure was varied between 40 and 53 mbar. Condenser temperature was set at 2 °C and rotor speed was constant at 200 rpm.
The first molecular distillation was made feeding essential oil, using an operating pressure of 53 mbar and obtaining as products the first residue (R1) and distillate (D1). Subsequent distillations were made feeding the residue obtained in previous distillation.
Table 2 shows the results of the three stages molecular distillations. Operation pressures and the obtained residue and distillate mass percentages are shown. In Table 2, %D and %R are defined as:

Table 2. Results of molecular distillation. (Rotor velocity: 200 rpm, Tcond=2°C, Tevap= 24 °C).
Distillate percentage decreases in successive distillations because fewer volatile components remain in the feeding mixture.
In the DPPH* assay used for the evaluation of free radical scavenging properties of the essential oil and its fractions, all the samples were able to reduce purple-colored stable radical DPPH* to yellow DPPH-H form. Fig. 4 shows the procedure followed to determine the IC50 value for oregano essential oil.
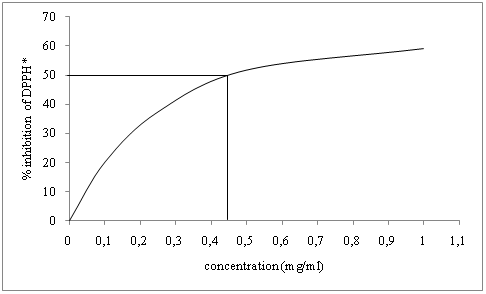
Fig. 4. IC50 determination for oregano essential oil
The results of the DPPH* technique on different stages of molecular distillation are shown in Fig. 5. IC50 for oregano essential oil was 0.45 mg.ml-1. All residues showed lower IC50 values, revealing an increase in their antioxidant potential. On the contrary, distillates showed a loss in their free radical scavenging properties with consequent increase of the IC50 value.
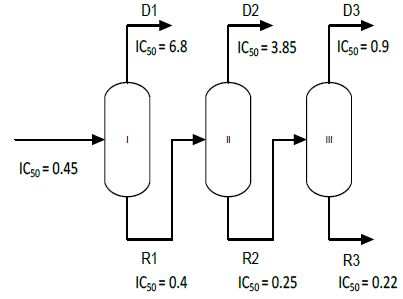
Fig. 5. Molecular distillations scheme
It is observed that both, the percentages of residue and distillate obtained and the IC50 value of these fractions, are not only function of the operating conditions of the distillation, but also of the composition of the raw material. However, it is observed that generally, the residue fractions become concentrated in antioxidant properties after molecular distillation.
In order to compare the free radical scavenging properties of the fractions studied with a commercial antioxidant, Table 3 shows IC50 values of the following samples: oregano essential oil, R3 (the fraction with higher antioxidant capacity) and BHT.
Table 3. IC50 values of oregano essential oil, R3 and commercial antioxidant BHT.

All the means of IC50 values were significantly different at the 5% level, except for R2 and R3. Figure 6 shows % Inhibition of DPPH* of the oregano essential oil, R2, R3 and BHT.
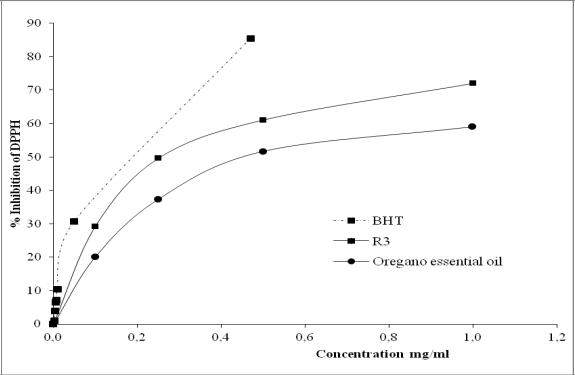
Fig. 6. Free radical scavenging properties of oregano essential oil, R3 and commercial antioxidant (BHT).
Table 3 shows that oregano essential oil concentrated by molecular distillation in the residue fraction has a significant antioxidant capacity.
Thymol was concentrated in all the residue fractions, probably increasing its free radical scavenging properties. This conclusion is based in the results of Kulisic et al. (2004), who demonstrated the important antioxidant capacity of this compound. Figure 6 shows the same tendency in the antioxidant properties for the different concentrations analyzed.
The detected components in oregano oil and its fractions, obtained by molecular distillation, are listed in Table 4. The principal components in oregano oil are trans-sabinene hydrate (27.506%) and the phenolic monoterpene thymol (16.318%). Figure 7 shows a typical oregano essential oil chromatogram.
Table 4. Components identified in oregano essential oil and its fractions.
Fig. 7. Typical oregano essential oil chromatogram
III. CONCLUSIONS
The residues obtained by molecular distillation of oregano essential oil were concentrated in antioxidant properties. Antioxidant power was evaluated by a free radical scavenging method, using the stable radical DPPH* as reagent, and determining the parameter IC50.
Variations in the operating pressure modified the evaporation efficiency, obtaining higher percentage of distillate at lower pressure.
In multistage distillation, distillate percentage decreases in successive distillations. The distillate percentage decreased even when the operation pressure was lower. This is probably due to fewer volatile components remain in the feeding mixture.
All the samples analyzed showed free radical scavenging capacity. The sample composition influenced the antioxidant properties. Residue fractions obtained by molecular distillation showed lower IC50 values than essential oil IC50, indicating an increase in their antioxidant properties. On the contrary, distillate fractions showed higher IC50 values, indicating a loss in their antioxidant potential. Residues were concentrated in thymol, suggesting that free radical scavenging capacity might be due to the presence of this phenolic monoterpene. Deterpetenation of the oil also contributes to its concentration in oxygen derivatives which may have a synergic behavior, increasing the antioxidant properties of the residue fractions.
The comparison of the IC50 parameter of the essential oil and its fractions with the synthetic antioxidant BHT indicates that fractions with higher levels of thymol have IC50 values comparable to the BHT one. The fraction with higher antioxidant power (R3) reported an IC50 value close to the antioxidant BHT. These results suggest that fractions concentrated in thymol and other minor oxygen derivatives obtained by molecular distillation could be used as natural antioxidants in the food industry. The time required for the reduction of DPPH* radical to its form DPPH-H, was higher for the essential oil and its fractions in comparison with the corresponding time required for commercial antioxidant BHT.
REFERENCES
1. Bakkali, F., S. Averbeck, D. Averbeck and M. Idaomar, "Biological effects of essential oils-A review," Food and Chemical Toxicology, 46, 446-475 (2008).
2. Bozin, B., N. Mimica-Dukic, N. Simin and G. Anackov, "Characterization of the volatile composition of essential oils of some lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils," Journal of Agricultural and Food Chemistry, 54, 1822-1828 (2006).
3. Brand-Williams, W., M.E. Cuvelier and C. Berset, "Use of free radical method to evaluate antioxidant activity," Lebensm. Wiss. Technology, 28, 609-615 (1995).
4. Burt, S., "Essential oils: their antibacterial properties and potential applications in foods- a review," International Journal of Food Microbiology, 94, 223-253 (2004).
5. Dambolena, J., M. Zunino, E. Lucini, R. Olmedo, E. Banchio, P. Bima and J. Zygadlo, "Total phenolic content, radical scavenging properties, and essential oil composition of origanum species from different populations," Journal of Agricultural and Food Chemistry, 58, 1115-1120 (2010).
6. Hazzit, M., A. Baaliouamer, M.L. Faleiro and M.G. Miguel, "Composition of the essential oils of Thymus and Origanum species from Algeria and their antioxidant and antimicrobial activities," Journal of Agricultural and Food Chemistry, 54, 6314-6321 (2006).
7. Kulisic, T., A. Radonic, V. Katalinic and M. Milos, "Use of different methods for testing antioxidative activity of oregano essential oil," Food Chemistry, 85, 633-640 (2004).
8. Loizzo, M., F. Menichini, F. Conforti, R. Tundis, M. Bonesi, A. Saab, G. Statti, B. Cindio, P. Houghton, F. Menichini and N. Frega, "Chemical analysis, antioxidant, antiinflammatory and anticholinesterase activities of Origanum ehrenbergii Boiss and Origanum syriacum L. essential oils," Food Chemistry, 117,174-180 (2009).
9. Milos, M., J. Mastelic and I. Jerkovic, "Chemical composition and antioxidant effect of glycosidically bound volatile compounds from oregano (Origanum vulgare L. ssp. hirtum)," Food Chemistry, 71, 79-83 (2000).
10. Montgomery, D.C. and G.C. Runger, Probabilidad y estadística aplicadas a la ingeniería, McGraw-Hill Interamericana Editores, México (1996).
11. Plazas Tovar, L., M. Wolf Maciel, G. Ferreira Pinto, R. Maciel Filho and D. Ramalho Gomes, "Factorial design applied to concentrate bioactive component of Cymbopogon citratus essential oil using short path distillation," Chemical Engineering Research and Design, 88, 239-244 (2010).
12. Rossi, P., A. Willnecker, J. Berti, A. Borgarello, G. Mezza and M. Pramparo, "D-limonene and geranial fractionation from lemon essential oil by molecular distillation," Latin American Applied Research, 41, 81-85 (2011).
13. Shan, B., Cai, Y., Sun, M. and H. Corke, "Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents," Journal of Agricultural and Food Chemistry, 53, 7749-7759 (2005).
14. Tsimogiannis, D., M. Stavrakaki and V. Oreopoulou, "Isolation and characterization of antioxidant components from oregano (Origanum heracleoticum)," International Journal of Food Science and Technology, 41, 39-48 (2006).
15. Wang, S., Y. Gu, Q. Liu, Y. Yao, Z. Guo, Z. Luo and K. Cen, "Separation of bio-oil by molecular distillation," Fuel Processing Technology, 90, 738-745 (2009).
16. Wojdylo, A., J. Oszmianski and R. Czemerys, "Antioxidant activity and phenolic compounds in 32 selected herbs," Food Chemistry, 105, 940-949 (2007).
17. Zheng, W. and S.Y. Wang, "Antioxidant activity and phenolic compounds in selected herbs," Journal of Agricultural and Food Chemistry, 49, 5165-5170 (2001).
Received: August 21, 2012
Accepted: April 17, 2013
Recommended by Subject Editor: María Luján Ferreira












