Services on Demand
Article
Latin American applied research
On-line version ISSN 1851-8796
Lat. Am. appl. res. vol.44 no.1 Bahía Blanca Jan. 2014
Effect of the ibuprofen solubility in acetone and dichloromethane on the drug release profiles from PLGA microspheres
D. M. Aragón, J. E. Rosas and F. Martínez
Sección de Farmacotecnia, Departamento de Farmacia, Universidad Nacional de Colombia, Cra. 30 No. 45-03, Bogotá D.C., Colombia.
dmaragonn@unal.edu.co, jerosasp@unal.edu.co, fmartinezr@unal.edu.co
Abstract — Acetone and dichloromethane were used as organic solvent to prepare ibuprofen-loaded PLGA microspheres by the emulsion-solvent evaporation method. Some microspheres properties, such as microencapsulation efficiency and particle size, were affected by the organic solvent used. Depending on the organic solvent used microparticles obtained exhibited different controlled release profiles. In all cases it was extended up to 15 days. The obtained formulations did not exhibit zero- or first-order release kinetics and non-agreement with the Higuchi or Korsmeyer-Peppas models was found. On the other hand, the model proposed by Gallagher and Corrigan for PLGA systems described fully the drug dissolution processes from the microspheres obtained. A relationship between the ibuprofen solubility in both organic solvents studied and some parameters estimated for the dissolution model of the microparticles prepared with these solvents was also found. Thus, it could be propose that the drug solubility in different organic solvents affects the physical characteristics of microparticles and their dissolution profiles.
Keywords — Ibuprofen; Thermodynamic Functions; Dissolution Profile; PLGA Microparticles.
I. INTRODUCTION
Ibuprofen (IBP, Fig. 1) is one of compounds more used for development of nano and microparticles because of its pharmacological and therapeutic application as analgesic and anti-inflammatory drug. Due to the low solubility of this drug in aqueous media, IBP is placed in the group II of biopharmaceutical classification system (BCS) along with other drug candidates for design of modified release systems (Dressman and Reppas, 2000).
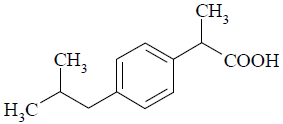
Figure 1. Molecular structure of ibuprofen.
Thus, using the technique of emulsification-solvent evaporation, it has been possible to develop polylactic acid (PLA) microspheres with yield up to 90% (Leo et al., 2000) In addition, by using poly-(D,L-lactic-co-glycolic acid) (PLGA) microparticles loaded with IBP, allowing an in vitro release profile extended by 8 days, have also been developed (Fernandez et al., 2004). Other polymers like polyvinylpyrrolidone (PVP) and poly-(methyl-methacrylate) (PMMA) have been used as surface modifiers in order to get different release profiles (Gallardo et al., 1998).
However, the development of these systems involves understanding and characterization of the different variables related to the drug, the polymer, and the microencapsulation technique. In a previous research, it has been observed that the particle size obtained is related to the polymer-solvent interaction parameter proposed by Flory and Huggins, which describes the affinity between any polymer and any solvent in terms of their respective Hildebrand solubility parameters and the molar volume of the solvent.
For the polymer PLGA (75:25) and four organic solvents it was observed that at higher polymer-solvent interaction parameter, smaller particle size is obtained (Choi et al., 2002). This kind of studies demonstrates the great importance of thermodynamic parameters of polymer-solvent interactions in the understanding of the micro and nano-encapsulation processes.
Similarly, other researches have demonstrated that the mean size and size distribution of the particles are determinate by the type of organic solvent and emulsion stabilizer used in the preparation of PLGA nanoparticles (Song et al., 2006).
Thus, the aim of this work was to evaluate the influence of the kind of organic solvents on the characteristics of IBP-loaded PLGA microparticles and the respective drug release behavior. In this way, it is a continuation of the one developed with the analgesic drug naproxen in similar conditions (Aragón et al., 2013).
II. MATERIALS AND METHODS
A. Materials
Ibuprofen (IBP) USP; Poly-(D,L-lactic-co-glycolic acid) PLGA 50:50 (Average molecular weight: 10,000-25,000 Da) Birmingham Polymers; Polyvinyl alcohol (PVA) 87-89% hydrolyzed (average molecular weight: 31,000 to 50,000 Da) Sigma-Aldrich; distilled water; dichloromethane (DCM) Merck; acetone (ACT) A.R Mallinckrodt; potassium chloride A.R. Scharlau; mono and disodium phosphate A.R. Merck; potassium chloride Merck A.R.; hydrochloric acid A.R. Merck.
B. Elaboration of Microspheres
For drug microencapsulation the emulsification-solvent evaporation method was used, which is recommended for drugs with low aqueous solubility as IBP is (Wischke and Schwendeman, 2008). This method has been previously described for other NSAIDs (Tuncay et al., 2000; Fernandez et al., 2004, Aragón et al., 2010a).
Briefly, the PLGA polymer and IBP (ratio 2:1) were dissolved in 5 mL the organic solvent studied (ACT or DCM), then the organic phase was emulsified with 50 mL the aqueous phase, containing polyvinyl alcohol (PVA, 4%) as stabilizing agent, by a homogenization process at 15,000 rpm for 2 minutes using an Ultraturrax® T-25. Once the emulsion was formed, it was kept under constant agitation for 2 hours in order to remove the organic solvent by evaporation, and therefore, to induce the formation of microparticles. The microparticles formed were separated by centrifugation, washed with distilled water, and finally freeze dried. All the microspheres were prepared by the same procedure in order to compare the organic solvent effect, which was changed according to the experimental design followed.
C. Characterization of Microspheres
C.1. Particle size and shape
The shape of the particles obtained was observed by scanning electron microscopy. The dry microparticles were coated with gold and examined with a scanning electron microscope (FEI QUANTA 200). The particle size was determined by light scattering (System Partikel-Technik, GmbH Sympatec).
C.2. Yield of the encapsulation process
The microparticles were weighed and the process yield was determined by using the Eq. 1.
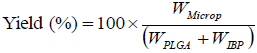 | (1) |
where WMicrop is the mass of the microparticles, WPLGA and WIBP are the masses of polymer and drug added, respectively.
C.3. Efficiency of encapsulation
The determination of the amount of microencapsulated drug and the efficiency of this process were carried out according to Fernandez et al. (2004). Briefly, 10 mg of microparticles were weighed and dissolved in 10 mL of DCM, later 10 mL of phosphate buffer pH 7.4 were added and the mixture was stirred continuously for three hours. After this time, the system was centrifuged and the IBP was quantified in the aqueous phase by UV spectrophotometry at 262 nm.
The encapsulation efficiency (%) was calculated from the relationship between the amount of drug incorporated in the microcapsules and the total initial amount of drug added during the microencapsulation process (Thomson et al., 2007) (Eq. 2).
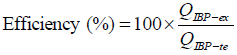 | (2) |
where QIBP-ex is the experimentally amount of drug obtained and QIBP-te the total amount of drug added.
C.4. Burst Effect
The drug release from polymeric microparticles can be described as a biphasic process. Initially a quick release (burst) phase occurs followed by a slower continuous release phase. The initial release plays an important role in the therapeutic efficacy and toxicity of microparticles and is very difficult to control. This effect is produced due to the drug quantity adsorbed at the particle surface, which release is independent of the polymer degradation.
The amount of IBP released from the microparticles in the first two hours, were evaluated by using a special test, according to Fernandez et al. (2004). 10 mg of microparticles were weighed and added to 10 mL of phosphate buffer pH 7.4 and stirred continuously for 2 hours. After this time, the aqueous medium was filtered and the drug release was quantified by spectrophotometry at 222 nm (Eq. 3).
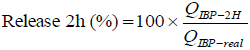 | (3) |
where QIBP-2H is the amount of drug released after 2 hours of agitation and QIBP-real is the amount of drug encapsulated.
D. Dissolution Test
Dissolution tests were carried out in microtubes, with 10 mg of microparticles and 1.0 mL of phosphate buffer pH 7.4. The tubes were placed on an orbital shaker microtube centrifuge at a speed of 20 RPM and incubated at 37 °C (Aragón et al., 2010a). At the times previously determined, the tubes were centrifuged at 10,000 RPM for 5 minutes, and then 200 μL of the supernatant solution were taken in order to measure the IBP released by using UV-analysis at 222 nm.
The cumulative amount of the released IBP was calculated considering the replaced volume of the dissolution medium. The cumulative percentage of the released drug was plotted versus time. Three samples of each one of the three batches of the two organic solvent were evaluated
E. Statistical Analysis
Results were expressed as the mean ± standard deviation of three batches of microparticles of each one of the two organic solvents studied. In order to evaluate the fit of the dissolution profiles of each one of the models proposed the statistical program STATGRAPHICS® PLUS 5.1 was used.
III. RESULTS AND DISCUSSION
It is well known that IBF microencapsulation by emulsification-solvent evaporation method involves several variables. For this reason, in order to evaluate only the effect of the organic solvent on the different properties under consideration, the microencapsulation methodology employed in this study was proposed based on some experimental data not shown here but reported previously (Aragón, 2009). In this way, by following that procedure, the selected conditions, i.e. drug : polymer ratio, volume of aqueous phase, stirring speed, and stirring time, are the ones reported in the "Materials and Methods" section.
With the selected variables, smooth and homogeneous PLGA microspheres loaded with IBF were obtained, regardless of the organic solvent used (Fig. 2). On the other hand, it is possible to observe the influence of the organic solvent on the yield of the microspheres, yielding values above 65% with DCM and near to 46% with ACT (Table 1). These results could be related to the equilibrium solubility of IBP in these solvents, especially in DCM, where in addition to the best yield, IBP also get the higher solubility and the most favorable Gibbs energy of mixing. Nevertheless, the similarity in solubility parameter values is more adequate in ACT (Table 2).
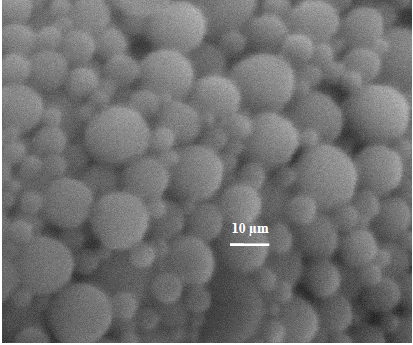
Figure 2. Surface morphology of PLGA microparticles loaded with IBP elaborated with ACT. Photo taken using SEM.
Table 1. Effect of organic solvent on the properties of PLGA microparticles loaded with IBP.
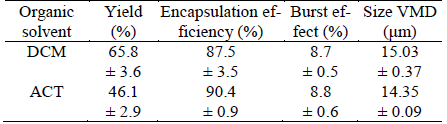
Table 2. Some physicochemical parameters of IBP, PLGA and organic solvents used
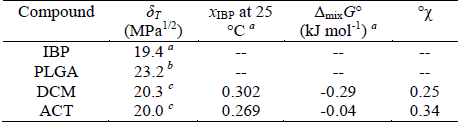
δT: total solubility parameter; °χ: polymer-solvent interaction parameter of Flory-Huggins. xIBP: experimental solubility for ibuprofen at 25 °C, expressed as mole fraction. a From Aragón et al. (2010b). b From Schenderlein et al. (2004). c From Barton (1991).
On the other hand, the thermodynamic reasons for the high solubility of IBP in both organic solvents studied have been reported previously in the literature (Aragón et al., 2010b). Briefly, this drug has low values of temperature and enthalpy of fusion which makes it easy to melt, from a hypothetical point of view, previously to mixing with the liquid solvent to give the saturated solution. On the other hand, solvent-solute interactions are favorable for IBP solubility due to hydrogen-bonding and London forces (Fig. 1). In particular, IBP would be acting as a Lewis acid in ACT and as a Lewis base in DCM.
It is also important to take into account the thermodynamic interactions between the PLGA and DCM or ACT, which can be expressed by the interaction parameter of Flory-Huggins (Sinko, 2006), which is calculated according to Eq. (4).
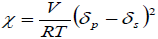 | (4) |
where V is the molar volume of solvent, R is the gas constant, T is temperature in K (in this case 298.15 K), δp is the solubility parameter of the polymer and δs is the solubility parameter of organic solvent. Thus, the greater the difference in solubility parameters between solvent and polymer, higher the Flory-Huggins parameter is. Table 2 presents some thermodynamic parameters of PLGA, IBP and both organic solvents used.
Previously it has been found that higher Flory-Huggins interaction parameter, smaller particle sizes are obtained (Song et al., 2006). In similar way, we obtained smaller particles when ACT was used as organic solvent. This fact can also be related to the poor yield found in this solvent, because the separation method used only allows large particles (greater than 10 μM). It is also important to note that ACT exhibit the greatest difference in the solubility parameter respect to PLGA but it is the nearest to IBP.
With respect to all of characteristics of the microparticles, there are not statistically significant differences between the results for the three batches prepared with both of the organic solvents showing good reproducibility of the microencapsulation method. On the other hand, there are some differences in the characteristic of the microparticles prepared with ACT and DCM (Table 1). In the case of the encapsulation efficiency values close to 90% in ACT and near to 87% in DCM were achieved. For the burst effect similar values close to 8.7% were found in both solvents.
In contrast to the behavior of naproxen in similar solvents (Aragón et al., 2013), the present results have not demonstrated an obvious influence of the organic solvent used in the preparation of PLGA microparticles loaded with IBP on their properties. Thus, the encapsulation efficiency is not influenced by the organic solvent used because the values obtained are not statistically different, whereas the yield is higher in DCM. Is important to note that DCM is the solvent where the experimental solubility of IBP is highest, as well as the Flory-Huggins interaction parameter is lowest, as was previously discussed. With respect to the burst effect, it could be said that no significant difference is found, as was already said. This fact could have a close relationship with the low amount of drug available on the surface of the particle.
A. Dissolution Profile of PLGA Microparticles Loaded with IBP
According to Fig. 3 a marked effect of the organic solvent used on the dissolution profiles of PLGA microparticles loaded with IBP was observed in the first 8 days (200 hours).
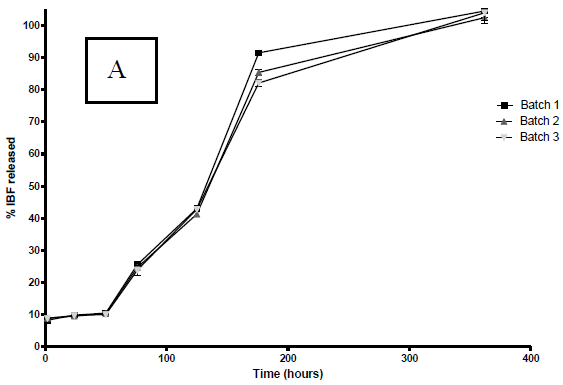
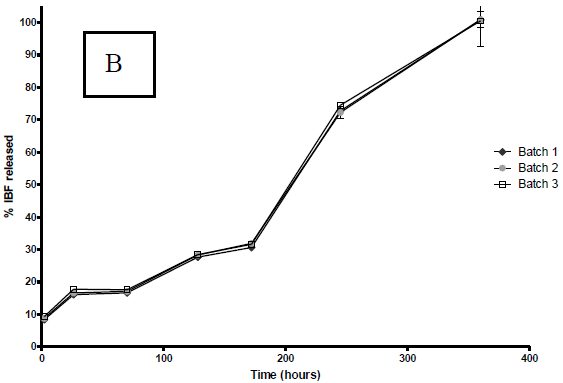
Figure 3. IBP release profiles from PLGA microparticles in buffer pH 7.4 at 37 °C. The profiles correspond to microparticles prepared with A: DCM; B: ACT. The graph shows the mean ± S.D. of each one of the three batches elaborated with each solvent.
Similar to the other variables studied, there is no apparent difference between each one of the three batches of microparticles prepared with the two solvents under consideration.
In all cases, the profiles obtained showed a maintained controlled release of IBP up to 360 hours (15 days). It is also possible to note that before the first 24 hours, there is a significant release of IBP varying from 10% in the case of DCM to 20% in the case of ACT. As well as the other characteristics, reflecting the reproducibility of the process, the three batches of microparticles prepared with each solvent, have similar release profiles.
In order to establish the kinetics and/or dissolution model which fit the results, the statistical program STATGRAPHICS® was used. The correlation degree between the variables of an established equation was also evaluated. With both solvents the determination coefficients found in the obtained profiles were below 0.20 when the equation for zero-order kinetics was applied and close to 0.90 when the equation for first-order kinetics was applied (Costa and Sousa, 2001).
Although there are higher determination coefficients for first-order kinetic, these results indicate that no one of these two types of kinetics describes clearly the IBP release behavior from PLGA microparticles. This fact was expected due to the great complexity of the drug release from PLGA polymer as was studied previously (Corrigan and Li, 2009; Puebla et al., 2005). In the same way, the data do not fit other dissolution models such as Higuchi model (Higuchi, 1961) or Peppas and Korsmeyer model (Korsmeyer et al., 1986), which have been successfully used in the description of the release profiles of many other controlled release dosage forms (Carriazo et al., 2010). In this study, the obtained values for the constants of the models previously mentioned are independent on the microparticles dissolution process. On the other hand, the dissolution profiles have a high degree of fitting to the models proposed by Gallagher and Corrigan (2000) for the release of drugs from PLGA microparticulate systems (Eq. 5). As it was did in a previous research (Aragón et al., 2013), we worked with the fraction of drug released and the time was expressed in days in order to analyze and compare the different results.
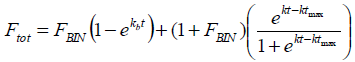 | (5) |
where FBIN is the burst fraction to infinite time and kb is a constant of first order associated with the release burst, which includes the diffusion coefficient and the solubility of the drug in the medium. The time with the highest rate of degradation of the polymer is represented by tmax and k is the respective constant.
This dissolution model has a high degree of correspondence with the experimental data. In both cases the determination coefficients were above 0.97, indicating that the model describes almost completely the release of IBF from the PLGA microparticles prepared and therefore, it is possible to infer mechanisms from the parameters found (Table 3). This model has also been successfully employed by other authors to describe the drug release from biodegradable dosage forms (Gallagher and Corrigan 2000; Millalos et al., 2008; Dunne et al., 2009) including micro- and nanoparticles (Gabor et al., 1999; Corrigan and Li, 2009).
Table 3. Estimated parameters for the IBP release from PLGA microparticles prepared from different solvents according to Equation 5.
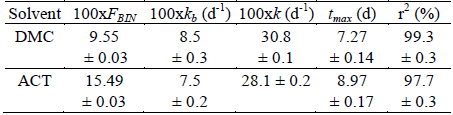
These results are expressed as mean ± SD of each one of the three batches elaborated with each solvent. d is days. FBIN represents the IBP released at the first 24 hours, these values are in agreement with those found in both experimental cases (Fig. 2) and they are lower than the FBIN values reported for other NSAIDs, such as naproxen from PLGA microparticles prepared in similar conditions, i.e. from 22.2 to 36.5 (Aragón et al., 2013). It is important to note that IBP solubility in ACT and DCM is greater than the naproxen solubility in the same solvents. On the other hand, the kb parameter represents the rate at which the drug diffuses into the bulk of the solution and is directly proportional to the surface area of the particle, the diffusion coefficient and the solubility of the drug in the dissolution medium. For the studied IBP microparticles there are no large differences in this parameter, as the solubility and diffusion coefficient are constant in all the tests developed. In this case, lower value of kb is presented for the microparticles prepared with ACT, which is the solvent with the higher value of interaction parameter of Flory-Huggins (Table 2).
Similarly, the tmax parameter represents the time when the rate of degradation of the PLGA polymer is maximum and therefore IBP has the highest rate of release. Although the particle size obtained in the particles prepared with DCM and with ACT is very similar, a direct relationship between tmax and the value of the polymer-solvent interaction parameter of Flory-Huggins was obtained in the present study.
Some relationship between the IBP solubility in both organic solvents and the k values was also found. k is related to the maximum rate of degradation of the polymer, as estimated for the dissolution profiles of the microparticles produced. Again, the solubility of IBP in the organic solvent used is related to release kinetics from PLGA microparticles. This fact has also been observed for naproxen with respect to several organic solvents analyzed (Aragón et al., 2013).
IV. CONCLUSIONS
From everything discussed previously we proposed that, despite of the fact that the solvent employed in the microencapsulation process of IBP into PLGA does not have influence in the microparticles properties, the drug release kinetic is clearly affected, and therefore, the in vivo behavior of the product could be affected. On the other hand, there is a relationship between the values of tmax and kb with the polymer-solvent interaction parameter of Flory-Huggins of PLGA in the solvents used. Finally, it is also possible to establish relationships between the IBP solubility in the organic solvents studied and some of the parameters estimated in the dissolution model.
ACKNOWLEDGEMENTS
We thank the DIB of the Universidad Nacional de Colombia (UNC) for the financial support. Additionally, we thank the Department of Pharmacy of UNC for facilitating the equipment and laboratories used.
REFERENCES
1. Aragón, D.M., Aspectos fisicoquímicos de la liberación de algunos analgésicos a partir de micropartículas de un polímero biodegradable, Ph.D. thesis, Universidad Nacional de Colombia, Bogotá D.C. (2009).
2. Aragón, D.M., N.E. Vergel, L.F. Ospina, F. Martínez and J.E. Rosas, "Effect of microencapsulated naproxen into poly(lactide-co-glycolide) microspheres on carrageenin paw edema in rats," Vitae, Rev. Fac. Quím. Farm., 17, 59-65 (2010a).
3. Aragón, D.M., J.E. Rosas and F. Martínez, "Thermodynamic study of the solubility of ibuprofen in acetone and dichloromethane," Braz. J. Pharm. Sci., 46, 227-235 (2010b).
4. Aragón, D.M., J.E. Rosas and F. Martínez, "Relationship between the solution thermodynamic properties of naproxen in organic solvents and their release profiles from PLGA microspheres," J. Microencapsulation, 30, 218-224 (2013).
5. Barton, A.F.M., Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd edition, CRC Press New York (1991).
6. Carriazo, D.A., M. Arco, C. Martín, C. Ramos and V. Rives, "Influence of the inorganic matrix nature on the sutained release of naproxen," Microporous and Mesoporous Materials, 130, 229-238 (2010).
7. Corrigan, O. and X. Li, "Quantifying drug release from PLGA nanoparticulates," Eur. J. Pharm. Sci., 37, 477-485 (2009).
8. Costa, P. and J.M. Sousa, "Modelling and comparison of dissolution profiles," Eur. J. Pharm. Sci., 13, 123-133 (2001).
9. Choi, S.W., H.Y. Kwon, W.S. Kim and J.H. Kim, "Thermodynamic parameters on poly(D,L-lactide-co-glycolide) particle size in emulsification-diffusion process," Coll. Surf. A: Phys. Eng. Asp., 201, 283-289 (2002).
10. Dressman, J. and C. Reppas, "In vitro-in vivo correlations for lipophilic, poorly water-soluble drugs," Eur. J. Pharm. Sci., 11, S73-S80 (2000).
11. Dunne, M., Z. Ramtoola and O.I. Corrigan, "Fluphenazine release from biodegradable microparticles: characterization and modelling of release," J. Microencapsulation, 26, 377-384 (2009).
12. Fernández, G., R. Herrero, I.T. Molina and P. Pastoriza, "Biodegradable ibuprofen-loaded PLGA microspheres for intraarticular administration. Effect of labrafil addition on release in vitro," Int. J. Pharm., 279, 33-41 (2004).
13. Gabor, F., B. Ertl, M. Wirth and R. Mallinger, "Ketoprofen-poly(d,l-lactic-co-glycolic acid) microspheres: influence of manufacturing parameters and type of polymer on the release characteristics," J. Microencapsulation, 16, 1-12 (1999).
14. Gallagher, K.M. and O.I. Corrigan, "Mechanistic aspects of the release of levamisole hydrochloride from biodegradable polymers," J. Control. Release, 69, 261-272 (2000).
15. Gallardo, J., L. Eguiburu, M. J. Fernandez, J. San Román, "Preparation and in vitro release studies of ibuprofen-loaded films and microspheres made from graft copolymers of poly(L-lactic acid) on acrylic backbones," J. Control. Release, 55, 171-179 (1998),
16. Higuchi, T., "Rate release of medicaments from ointment bases containing drug in suspension," J. Pharm. Sci., 50, 874-875 (1961).
17. Korsmeyer, R.W., S.R. Lusting and N.A. Peppas, "Solute and penetrant diffusion in swellable polymer. I. Mathematical modeling," J. Polym. Sci. B., Polym. Phys., 24, 395-408 (1986).
18. Leo, E., F. Forni, M.T. Bernabei, "Surface drug removal from ibuprofen-loaded PLA microspheres," Int. J. Pharm., 196, 1-9. (2000).
19. Milallos, R.G., K. Alexander and A. Riga, "Investigation of the interaction between acidic, basic, neutral and zwitterionic drugs with poly-l-lactic acid by thermal and analytical methods," J. Therm. Anal. Calorim., 93, 289-294 (2008).
20. Puebla, P., P. Pastoriza, E. Barcia and A. Fernandez-Carballido, "PEG derivative effectively modifies the characteristics of indomethacin-PLGA microspheres destined to intra-articular administration," J. Microencapsulation, 22, 793-808 (2005).
21. Schenderlein, S., M. Luck and B.W. Müller, "Partial solubility parameters of poly(d,l-lactide-co-glycolide)," Int. J. Pharm., 286, 19-26 (2004).
22. Sinko, P.J., Martin's Physical Pharmacy and Pharmaceutical Sciences, Lippincott Williams & Wilkins, Baltimore (2006)
23. Song, K.C., H.S. Lee, I.Y. Choung, K.I. Cho, Y. Ahnb and E.J. Choi, "The effect of type of organic phase solvents on the particle size of poly(d,l-lactide-co-glycolide)nanoparticles," Colloid. Surf. Physicochem. Eng. Aspect., 276, 162-167 (2006).
24. Thompson, C.J., D. Hansford, S. Higgins, C. Rostron, G.A. Hutcheon and D.L. Munday, "Evaluation of ibuprofen-loaded microspheres prepared from novel copolyesters," Int. J. Pharm., 329, 53-61 (2007).
25. Tuncay, M., S. Calis, H.S. Kas, M.T. Ercan, I. Peksoy and A.A. Hincal, "Diclofenac sodium incorporated PLGA (50:50) microspheres: formulation considerations and in vitro : in vivo evaluation," Int. J. Pharm., 195, 179-188 (2000).
26. Wischke, C. and S. Schwendeman, "Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles," Int. J. Pharm., 364, 298-327 (2008).
Received: October 4, 2012
Accepted: June 25, 2013
Recommended by Subject Editor: María Luján Ferreira