Servicios Personalizados
Articulo
Latin American applied research
versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.44 no.1 Bahía Blanca ene. 2014
Ultrasonic studies of antiprotozoal drug in protic and aprotic solvents at 308.15 K
S. Baluja†, A. Kulshrestha and M. Bhatt
Department of Chemistry, Saurashtra University, Rajkot-360005, (Gujarat), India.
† shipra_baluja@rediffmail.com
Abstract — Ultrasonic velocities and density of various concentrations (0.01 to 0.10M) of anti protozoal drog "Diloxanide Furoate" in methanol, dimethyl formamide and 1,4-dioxan have been measured at 308.15 K by using single crystal variable path ultrasonic interferometer operating at 2 MHz and pycnometer respectively. Using these experimental data, some acoustical parameters have been evaluated and they are interpreted in terms of solute-solute and solute-solvent interactions in these solutions.
Keywords — Anti Protozoal Drug; Ultrasonic Studies; Diloxanide Furoate.
I. INTRODUCTION
Diloxanide furoate is a luminal amebicide drug used in the treatment of amebiasis (Fernandes et al., 2009). It is a dichloro acetamide derivative that principally acts in the bowel lumen and is used in the treatment of intestinal amoebicide (Reynolds, 2005). In addition to its anti-protozoal and anti-amoebic effect (Abbas et al., 2011), the drug contains an ester group which has wide usage in flavoring, perfumery, artificial essences, cosmetics, etc. (Abdelaleem and Abdelwahab, 2012).
These properties prompted us to study the molecular interaction of Diloxanide furoate in some protic and aprotic solvents. Ultrasonic velocity measurements in solutions furnish knowledge about ion-ion and ion-solvent interactions.
In the last few years, our investigation groups have carried out some studies on acoustical properties of organic compounds in various solvents (Baluja and Parsania, 1995; Baluja and Oza 2003; Baluja and Shah, 2004, Baluja et al., 2005; Baluja et al., 2009) as well as on some drugs (Godvani et al., 2010; Godvani et al., 2012 ).
In continuation of these investigations, the present paper reports acoustical properties of the anti- protozoal drug "Diloxanide Furoate" in different solvents.
II. EXPERIMENTAL
The solvents methanol, DMF and 1,4-dioxan used in the present work were of AR grade and purified according to the standard procedure (Riddick, 1986). The purity of solvents was checked by GC-MS and was found to be more than 99.97%.
The densities of pure solvents and their solutions were measured by using a single capillary pycnometer, made of borosilicate glass having a bulb capacity of 10 ml. The ultrasonic velocity of pure solvents and their solutions were measured by using a single crystal variable path ultrasonic interferometer operating at 2 MHz. The accuracy of density and velocity are ± 0.0001 g/cm3 and ± 0.1% cm/sec respectively. All the measurements were carried out at 308.15 K. The uncertainty of temperature is ± 0.1 K and of concentration is 0.0001 moles /dm3.
III. THEORY
From the experimental data of density (ρ) and ultrasound velocity (U) of pure solvent and solutions, various acoustical parameters were calculated using following standard equations (Baluja and Oza, 2003).
Isentropic compressibility (κS):
κS = 1/ (U2ρ) (1)
Intermolecular length (Lf):
Lf = KJ κS ½ (2)
where KJ is Jacobson constant (= 6.0816 x 104).
Rao's molar sound function (Rm):
Rm= (M/ρ)U1/3 (3)
where M is the molecular weight of solution.
Van der Waal's Constant (b):
b = (M/ρ) (1-RT/MU2 (√ (1+MU2/3RT)-1)) (4)
where R is gas constant and T is absolute temperature.
Molar Compressibility (W):
W = (M/ρ) κS-1/7 (5)
Solvation number (Sn) :
Sn = M2/M1 [1- κS / κS1] [(100- X) /X] (6)
where X is the number of grams of solute in 100 gm of the solution. M1 and M2 are the molecular weights and κS1 and κS are isentropic compressibility of solvent and solute respectively.
IV. RESULTS AND DISCUSSION
Table 1 shows the experimental data of density and ultrasonic velocity (U) of solutions of the anti-protozoal drug at 308.15 K in different solvents; methanol, DMF and 1,4-dioxane. It is observed that in all the three selected solvents, density as well as ultrasonic velocity increase with concentration.
Table-1:- Some acoustical parameters of the drug in methanol, DMF and 1, 4 Dioxan at 308.15 K
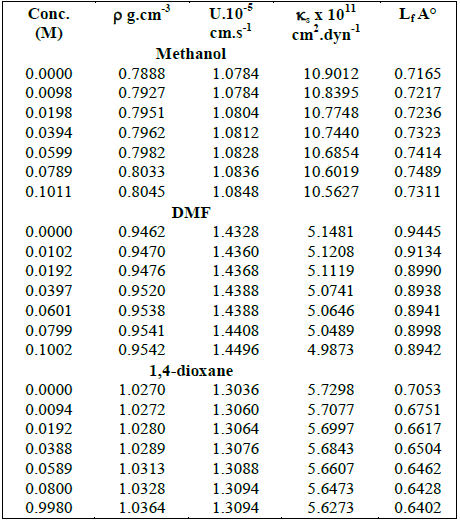
Further, intermolecular free length (Lf) and isentropic compressibility (κs) values are also reported in Table 1. Both Lf and κs decreases with the increase in concentration for all the solutions.
The increase of U and decrease of κs and Lf suggest aggregation of solvent molecules around solute molecules indicating thereby the presence of solute-solvent interactions.
Figure 1 shows the variation of some acoustical properties like molar sound function (Rm), molar compressibility (W) and Van der Waals constant (b) with concentration. These parameters are observed to increase linearly (correlation coefficient γ=0.9997 - 0.9999) with the increase of concentration for all the solutions. The linear variation of these acoustical properties indicates the absence of complexes formation in these systems.
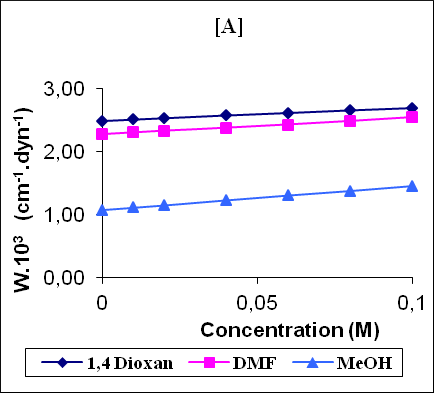
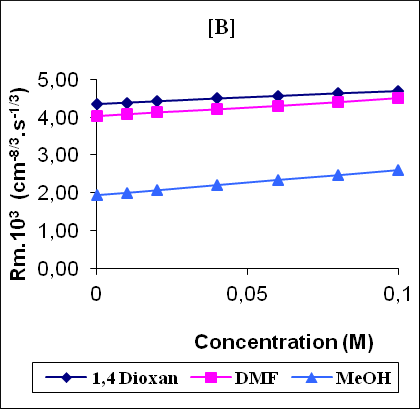
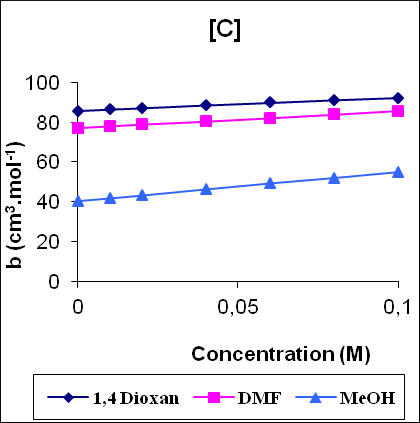
Figure 1: The variation of molar compressibility W(A), molar sound function Rm (B) and Vander Waals constant b (C) with concentration.
Figure 2 shows the variation of solvation number (Sn) with concentration. The solvation number (Sn) is a measure of structure-forming or structure-breaking tendency of a solute in solutions. In all the systems, Sn values increases non-linearly with the increase of concentration, which suggests structure-forming tendency of the drug in these solvents.
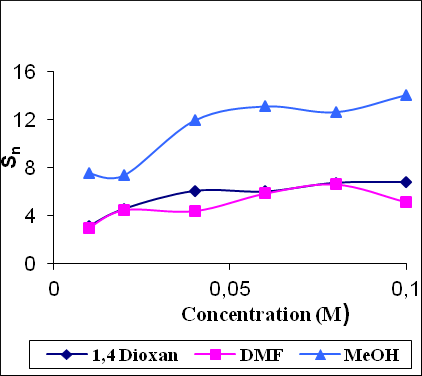
Figure 2: Variation of solvation number (Sn) with concentration in different solvents.
V. CONCLUSION
It is concluded that, in the studied solvents, the drug exhibited predominance of solute-solvent interactions. Therefore, structure-forming tendency of the drug is observed in the studied solvents and it has a maximum in methanol. The effect of concentration on solute-solvent interaction is also higher in methanol and minimum in DMF solutions. Further, there is no complexes formation in the studied solutions.
ACKNOWLEDGEMENT
Authors are thankful to Head of Chemistry Department for providing facilities
REFERENCES
1. Abbas, S.S., N.E. Wagieh, M. Abdelkawy and M.M. Abdelrehman, "Simultaneous determination of diloxanide furoate and metronidazole in presence of diloxanide furoate degradation products," JAOAC Int., 4, 1427-1439 (2011).
2. Abdelaleem, E.A and N.S. Abdelwahab, "Simultaneous determination of some antiprotozoal drugs in different combined dosage forms by mean centering of ratio spectra and multivariate calibration with model updating methods," Chem Cent J., 6, 27-34 (2012).
3. Baluja, S. and P.H. Parsania, "Acoustical properties of 3-a-Furyl acrylic acid in protic and aprotic solvents," Asian J. Chem., 7, 417-423(1995).
4. Baluja, S. and S. Oza, "Ultrasonic studies of some derivatives of 6-ethylbenzene-1,3-diol in 1,4-dioxane," Fluid Phase Equil.,208, 83-89 (2003).
5. Baluja, S. and A. Shah, "Acoustical studies of some derivatives of 4-amino antipyrene in 1,4-dioxane and dimethylformamide at 318.15 K," Fluid Phase Equil., 215, 55-59 (2004).
6. Baluja, S., N. Pandaya, N. Kachhadia, A. Solanki and P. Inamdar, "Thermodynamic and Acoustical studies of binary mixtures of diethyl malonate at 308.15 K," Phys. Chem. Liq., 43, 309-316 (2005).
7. Baluja, S., R. Bhalodia and R. Gajera, "Synthesis and ultrasonic studies of some dihydro pyrimidines in different solvents at 298.15 K," Int. J. Appl. Chem., 5, 47-55 (2009).
8. Fernandes, H, C.R. D'Souza, G.K. Swethadri and C.N. Naik, "Ameboma of the colon with amebic liver abscess mimicking metastatic colon cancer," Ind. J Pathol Microbiol, 52, 228-30 (2009).
9. Godvani, N., J. Movalia, R. Gajera and S. Baluja, "Study of molecular interactions of Loperamide drug in different solvents at 308.15 K," Res. J. Pharma. Bio. Chem. Sci., 1, 67-73 (2010).
10. Godvani, N., J. Javiya, J. Movaliya and S. Baluja, "Studies of Molecular Interactions In Solutions of Phenylephrine Drug At 308.15K," Asian J. Biochem. Pharma. Res., 2, 131-139 (2012).
11. Reynolds, J.E.F., Martindale: Extra Pharmacopoeia, 34th Ed., The pharmaceutical Press, London, UK (2005).
12. Riddick, J.A., W.B. Bunger and T. Sakano, Organic Solvents-Physical Properties and methods of purification, Fourth Edition., Techniques of Chemistry, II, A Wiley-Interscience Publication, John Wiley, New York (1986).
Received: July 16, 2012
Accepted: December 28, 2012
Recommended by Subject Editor: María Luján Ferreira












