Servicios Personalizados
Articulo
Latin American applied research
versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.44 no.2 Bahía Blanca abr. 2014
Study of a pilot plant for the recovery of metals from spent alkaline and zinc-carbon batteries with biological sulphuric acid and polythionate production
L. Falco† - A. Martínez† - M. P. Di Nanno‡ - H. Thomas† and G. Curutchet‡
† Pla.Pi.Mu. Comisión de Investigaciones Científicas Prov. De Bs. As.- Universidad Nacional de La Plata, La Plata CP1900, Argentina. mlfalco@quimica.unlp.edu.ar
‡ Centro de Estudios Ambientales. Escuela de Ciencia y Tecnología, Universidad Nacional de San Martín, San Martín, Argentina. gcuruche@unsam.edu.ar
Abstract— The recovery of Zn and Mn from spent alkaline and Zn-C batteries with a biohydrometallurgycal process was studied in a pilot plant that consists of an air-lift bioreactor with a sulphur packed bed where Acidithiobacillus thiooxidans produces an acid-reducing medium; a leaching reactor where the acid-reducing medium is mixed with the battery powder, and a recovery reactor where metals are recovered from the leaching liquor by electrolysis. Results show that with acid medium (350 mM[H+]) produced in 12 days by Acidithiobacillus thiooxidans in the bioreactor, an extraction of 100% of Zn and 67% of the Mn present in the battery powder was reached. The presence of polythionates in the medium produced in the bioreactor allows the dissolution of the manganese. The solid remaining after bioleaching is a manganese oxide. The electrolysis of the leaching liquor produced a cathodic deposit of metallic Zn and an anodic deposit of a manganese oxide in one step at room temperature.
Keywords— Batteries; Biohydrometallurgy; Acidithiobacillus Thiooxidans; Metal; Recovery.
I. INTRODUCTION
There are several approaches at the international level to address the problem of spent batteries, though none of them has been universally accepted. There are different alternatives to the final destination of batteries: landfill, stabilization, incineration or recycling (Bernardes et al., 2004). In the last years many technologies for battery recycling have been developed in several countries, such as pyrometallurgy, acid leaching, alkaline leaching, combined acid-reducing leaching, solvent extraction, electrolysis and chemical precipitation (Bernardes et al., 2004, Provazi et al., 2011). Many patented processes have already been applied mainly for the treatment of dry cell batteries such as BATENUS for mixed batteries, PLACID for mercury recovery, RECYTEC for the simultaneous recovery of zinc and manganese dioxide, HYDROMETAL SPA for lead-acid batteries, REVABAT/REVATECH for alkaline and zinc-carbon batteries, RECUPYL for all types of batteries (Ferella et al., 2008; Sayilgan et al., 2009a). EcoRecycling SRL and the University La Sapienza of Rome have patented the first recycling plant of spent alkaline and zinc-carbon batteries in Italy (Toro et al., 2011). According to the European Union Battery Directive Extended Impact Assessment report, each year, approximately 800,000 tonnes of automotive batteries, 190,000 tonnes of industrial batteries and 160,000 tonnes of portable batteries are placed on the community market. The total weight of portable batteries sold in Eastern and Western Europe in 2003 was about 164,000 tonnes, of which 50,197 and 99,138 tonnes were zinc-carbon and alkaline batteries, respectively (30.5% and 60.3% of total annual sales) (Sayilgan et al., 2009a; EPBA, 2006). Xin et al. (2012a) reported that Zn-Mn batteries account for over 90% of the total annual sales of portable batteries due to their low prices, especially in developing countries like China. Legislation in many countries regulates the fabrication, commerce and final disposal of spent batteries; in the European Union and USA, there are already industrial plants for battery recycling. In Latin America, there are some new regulations on the fabrication and final disposal of batteries, but there is still a lot to do about recycling. In Argentina, in particular, spent batteries are sent to the landfills with the domestic garbage (European Comunities, 2006; Argentina, 2006; Espinosa et al., 2004). Spent batteries represent a valuable resource, since this kind of waste contains high levels of metals whose prices are rising worldwide. The benefits of recycling materials from an economic, environmental and technical point of view depend on many factors, including transport, recycling process and material to be treated. Recycled nickel and cadmium, for example, require 46% and 75% less primary energy, respectively, than the extraction and refining of the virgin metal (Ridh and Karlstrom, 2002). For zinc, the relation between the energy needed for recycling and the energy needed for extraction from primary resources is 2.2 to 8. These figures are particularly important given the fact that the primary production of metals is the source of approximately 10% of global CO2 emissions (European Comunities, 2003).
In this work, a biohydrometallurgical process for the recovery of metals from spent batteries is proposed. Biohydrometallurgy can be defined as the field of application resulting from the control of natural (biochemical) processes of interactions between microbes and minerals to recover valuable metals (Morin et al., 2006). Biohydrometallurgycal processes are a robust emerging technology with some advantages over pyrometallurgical systems and chemical leaching processes, namely, less energy consumption, less atmospheric emissions, small, safe and versatile plants, simplicity and low cost of the process, applicability to low- grade sources , low costs of installation and possibility of on-site treatment (Morin et al., 2006; Brierley and Brierley, 2001, Brierley, 2010). In the field of biohydrometallurgy, three different processes can be defined: bioleaching, bio-oxidation and indirect acid bioleaching using acid production by acidophilic bacteria cultivated in bioreactors or bioheaps. In the last few years several studies on the bioleaching of metals from solid waste have been reported (Cerruti et al., 1998; Bosio et al., 2008; Zhao et al., 2008; Xin et al., 2009; Xiang et al., 2010). Cerruti et al. (1998) studied the bioleaching of spent Ni-Cd batteries with Acidithiobacillus thiooxidans. Previous studies, at laboratory scale, dealt with the bioleaching of spent alkaline batteries (Xin et al., 2012a; 2012b) and Li-ion batteries (Xin et al., 2009) with acidophilus bacteria such as Alicyclobacillus sp. (sulphur-oxidizing bacteria) and Sulfobacillus sp. (iron-oxidizing bacteria). However, no pilot plant scale studies of biohydrometallurgy for battery recycling have been found in the literature. On-site sulphuric acid production has multiple advantages, since it eliminates the manipulation of concentrated sulphuric acid, the pollution of its industrial production and the costs of transporting it (Brierley, 2010). Despite the extensive literature on the bioleaching of sulphide metals and arsenopyritical gold, only a few publications are dedicated to the biologic production of sulphuric acid and intermediate sulphur compounds for industrial applications. Acidithiobacillus thiooxidans (At) is a chemolitotrophic bacterium able to catalyse the oxidation of elemental sulphur and other reduced sulphur compounds to polythionates and sulphuric acid, and to use it as its energy source. It requires a minimal mineral medium and is easily cultivated in the laboratory. It has a remarkable tolerance to heavy metals and low pHs.
In this paper a pilot plant for the bioleaching of spent alkaline and zinc-C batteries was studied. This pilot plant consists of a packed bed air-lift bioreactor with attached At cells for acid and polythionate production, a bioleaching reactor where metals are extracted from the battery powder with the acid-reducing medium produced in the bioreactor, and a recovery reactor where Zn and Mn are recovered from the leaching liquor by electrolysis. The advantages of this indirect bioleaching are the possible optimisation of the acid production in one reactor and the leaching of metals in the other. This indirect mechanism also makes it unnecessary to adapt the cells to high metal concentrations.
II. METHODS
A. Process flow sheet
In Fig. 1 there is a scheme of the pilot plant for the recovery of metals from spent batteries. The batteries are opened and the steel case, plastic and paper are separated. The battery powder is washed several times with water in order to eliminate the electrolyte. In the air-lift bioreactor with a sulphur- packed bed, At produces sulphuric acid and polythionates. This product is mixed with the battery powder in the bioleaching reactor, which is a stirred-tank reactor. The resulting leaching solution is filtered and sent to the recovery reactor, which is an electrolytic cube where metallic zinc and a manganese oxide are deposited simultaneously. The solid remaining after the leaching is a manganese oxide. The basic electrolyte, separated in the washing step, can be used for the neutralization of the liquid acid residue after metal electrodeposition.
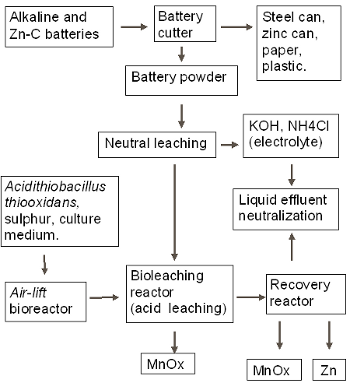
Figure 1.Process flow sheet.
B. Bacteria
An Acidithiobacillus thiooxidans (At) strain DSM 11478 was used. The organism was routinely maintained in a minimum mineral medium (1g/L SO4(NH4)2, 0.5g/L K2HPO4, 0.5g/L MgSO4.7H2O, 0.0145 g/L CaCl2.2H2O, 0.1 g/L ClK). The pH was initially adjusted to 2.5 and sulphur powder was added as the energy source (1% m/V). The organism was cultured in Erlenmeyer flasks and incubated at 30deg;C on a rotary shaker at 180 rpm.
C. Sulphuric acid and polythionate production
The experiments were carried out using the reactor shown in Fig.2. The air-lift reactor used was built of acrylic, 3 mm width. Inside the downcomer section, sulphur (+3.5; -5 mesh) was added. This sulphur constituted the support media and the substrate for At. The gaseous phase was air in every experiment. Airflows ranging from 75 to 240 L/h were fed continually to the reactor by the riser section. The reactor was maintained at 30deg;C by a temperature control system. The total volume of the reactor was 26.5 L. The reactor was inoculated once with a culture of At in the exponential stage of growth. When proton concentration reached the settled value, the same volume of fresh mineral medium without new inoculation replaced the entire medium. This acidified medium was used afterwards to leach the metals contained in the batteries.
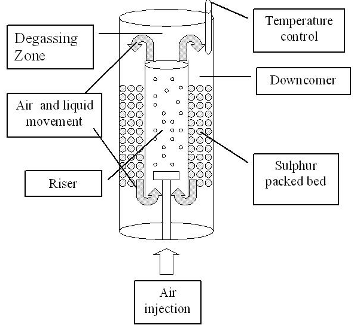
Figure 2. Air-lift bioreactor scheme.
To characterise the reactor we evaluated the oxygen volumetric transfer coefficient (KLa, Sulphite method; Bullock and Kristiansen, 1995). Periodically, samples were taken to evaluate proton (titration with NaOH ) and sulphate concentration (turbidimetric method), pH (potentiometric), polythionates (UV spectrophotometry (Shirihari et al., 1993), and cells/mL (direct counting, Burker chamber). The sulphuric acid/sulphur yield was calculated weighing sulphur before and after a certain amount of acid production. Here, the cell mass adhered to the sulphur surface is considered negligible (Konishi et al., 1995). We define every time new liquid medium is added as a step in the reactor.
D. Batteries
A mixture of Zn-C and alkaline batteries, size AA, from different trademarks were used in this work. They are primary cells, so they can be fully discharged once and then discarded. Their principal components are zinc, manganese dioxide, NH4Cl or KOH electrolyte and a steel case (Energizer, 2001, 2008).
E. Battery pre-treatment
Batteries are cut by the battery cutter (equipment specially designed in our laboratory) and opened. The battery powder is separated from the case as well as all the ferrous parts, plastic and paper. The powder is washed with several portions of distilled water to eliminate the electrolyte, and then dried at 150deg; overnight.
F. Bioleaching of batteries
Spent alkaline and zinc-carbon battery powders are treated with the biological acid-reducing medium to transfer the metals from the solid to the leaching liquor. The bioleaching experiments were carried on a 50 L polypropylene reactor mechanically stirred by a marine propeller at 200 rpm. Then, 2 kg of battery powder was placed in the leaching reactor with 28 L of acid medium (pH near 0.4). When the pH reached a plateau and metals in solution did not increase, the entire medium was replaced by fresh acid medium in order to continue metal dissolution, as necessary.
The steel case extraction avoids excess Fe in solution and allows the direct recovery of steel for the iron and steel industry, as it reduces costs in acid consumption and pH control. Periodically, samples were taken from the reactor to analyse metal concentration (atomic absorption in a Perkin Elmer 3110 spectrophotometer) and pH.
To evaluate if there was any difference between the metal extraction done by a commercial or biological sulphuric acid, in one Erlenmeyer flask, named S1, the battery powder was placed in contact with commercial sulphuric acid at the same concentration as the biological one, and in another flask, named S2, the battery powder was put in contact with biological acid. Samples were taken periodically for pH and metal concentration determination.
G. Electrolysis
A sample of 6.5 L of the leaching solution was transferred from the bioleaching reactor to an electrolytic cube with two stainless steel electrodes connected to a DC power source. The average current density was 100 A/m2 and the distance between electrodes was 25 cm. Experiments were carried at atmospheric pressure and room temperature, taking samples of the electrolytic bath to determine metal concentration by atomic absorption and pH with a combined electrode. The solid deposited in the electrodes was taken at the end of the experiment and characterised as we describe below.
H. Electrolytic MnOx, Zn, battery powder, and residual leaching solid characterisation
The metal composition of all the solid samples was determined by acid digestion with HCl-HNO3 (3:1) and atomic absorption in Perkin Elmer model 3110 spectrophotometer with the corresponding hollow cathode lamp. The BET surface areas of the samples were determined by nitrogen adsorption at 77 K using a Micrometrics ASAP 2020 analyser. Crystal structures of the samples were analysed by X-ray diffraction methods in a Philips Diffractometer using Cu Kα (λ = 1.5406 A) radiation at 2 °min-1 scanning speed, in the range 5<2θ<70°. The surface morphology and quantitative analysis of the composition of the samples were carried out with a scanning electron microscope provided with energy dispersive X-ray analysis (SEM-EDS) using a Philips SEM 505 microscope.
III. RESULTS AND DISCUSSION
A. Sulphuric acid and polythionate production
a. Biofilm development in the packed bed reactor
Proton concentration (mM) as a function of time is shown in Fig. 3 for seven different steps and the same cycle (75 L/h airflow and 8.2 kg sulphur, end point of each step: 150 mM proton approximately). In the curves we might appreciate different phases: lag, exponential and lineal in agreement with previous results (Ceskova et al.,2002). The short exponential phase followed by a lineal phase suggests a kinetic limitation by gaseous nutrients transfer or by free sulphur sites. The curves move to the left in the successive steps without appreciable changes in their slopes. The reduction of the lag phase from 264 to 72 hours in four steps is probably due to the biofilm formation in the sulphur pearls. The proton production curves might be assimilated to total biomass growth. Saturation of the available sulphur sites is probably reached from the fourth charge, because we do not observe higher increases in proton productivity. The volumetric proton productivity (generated proton per unit of time and volume) increases as the lag phase decreases, reaching values between 13.4 and 16.8 mM/day in this cycle.
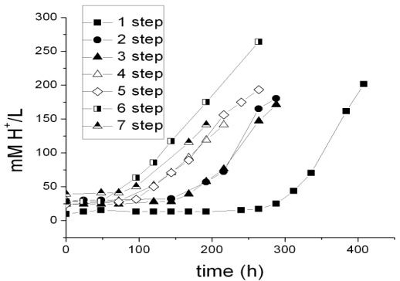
Figure 3. Production of acid by At. in the bioreactor
Proton volumetric production as a function of sulphur mass
We evaluated proton productivity for two different sulphur mass and liquid media relations, namely 410 and 815 sulphur g/L, with a constant airflow of 75 L/h. When we used 410 g/L we obtained a productivity of 14.6 ± 1.5 mM proton/day (the error is the standard deviation for n=4, n representing steps in this case) and for the second relation a 47% better value of 27.7 ± 2.5 (n=2) mM proton/day. But it must be considered that an increase in sulphur mass diminishes the space available for the mineral medium (20 L for the first, 13 L for the second). Taking this into account, there is a net increase of 23% in the acid production (millimol proton/day) for a 50% increase in sulphur/medium relation.
Proton volumetric productivity as a function of the oxygen volumetric mass transfer coefficient (KLa)
Between 75 and 240 L/h airflow we observed a linear relationship with KLa. This indicates that we might further increase the airflow to obtain higher KLa values. The KLa value indicates oxygen transfer in the reactor. At is an aerobic microorganism that requires oxygen as an electron acceptor, so the culture might become limited in this transfer. To evaluate this limitation we correlated KLa with proton productivity in the range studied. We observed no increase in acid productivity over 138 L/h airflow in spite of the increase of KLa. This indicates that over a KLa value of 10.4 ± 0.8 L/h the system is no longer limited by oxygen transfer, so there is no reason to increase airflow and costs. However, in the oxygen limited zone we obtained a 30% increase in proton productivity by raising the airflow between 75 and 138 L/h (29.9 ± 2.5 to 42.3 ± 0.6 proton mM/day).
Sulphuric acid/sulphur yield (YA/s) as a function of the end point of the batch culture
Polythionates (Sn(SO3)2-2) in water decompose and their solutions must be stabilised by adding OH-. They are the intermediate products of the oxidation of sulphur catalysed by At. When the batch culture is finished, they are present in the acid media (see Fig. 4). If they are not readily oxidised, more sulphur is consumed than that stoichiometrically required to reach that level of acidity.
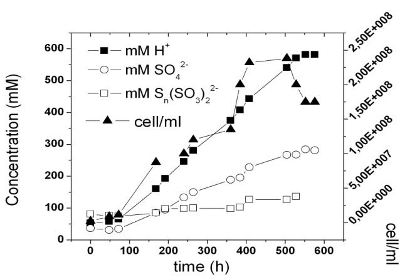
Figure 4. Sulphuric acid and polythionate production and At. free cell evolution in the air-lift reactor.
We studied the sulphuric acid/sulphur yield varying the end point of the batch culture (150 or 350 proton mM). This parameter is important since sulphur consumption is one of the highest costs for the biological production of this acid (the other is energy for heating, but this can be solved by modifying some design parameters).
As At might become inhibited by the product -though they resist high levels of acidity and sulphate- we evaluated a number of free cells in a culture that has reached a constant acidity level.
n this test we found increases in cell population until 540 proton mM and 260 mM of sulphate (Fig. 4). Overthat concentration, cells begin to disappear. The final proton concentration stabilises at 600 mM approximately (pH= 0.2). Using these results we selected lower proton concentrations for our experiments. The theoretical YA/S is 3.0625 g/g (98 g sulphuric acid/32 g sulphur). Using an end point concentration of 150 proton mM we obtained a yield of 0.54, while by incrementing the proton concentration to 350 mM, we increased this yield to 2.07 g/g.
B. Bioleaching
Determination of battery powder composition
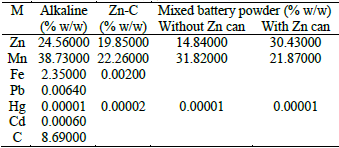
Acid digestion and atomic absorption spectrophotometry: Battery powder composition before leaching is quite different depending on the trademark; electrolytes are in general K(OH) or NH4Cl, which were eliminated after washing with distilled water. See Table 1.
Table 1. Battery powder composition
SEM-EDS: In Fig. 5 it can be seen that the most important components of the battery powder are Zn and Mn; a small quantity of Ni is also present, probably coming from the stainless steel case, and K from the electrolyte.
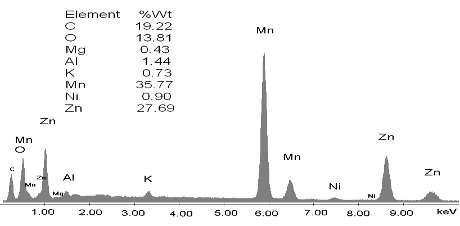
Figure 5.Battery powder EDS spectra before leaching.
Leaching reactor
In two successive extractions it was observed that Zn is easier to extract than Mn, zinc and zinc oxide are totally dissolved by sulphuric acid, eq. (1) and (2), while manganese oxides are only partially leached by sulphuric acid, see Eqs. (3-5) (Sayilgan et al.,2009b; Ferella et al., 2010). But the leaching media produced by At in the bioreactor is composed of sulphuric acid and polythio-nates as intermediate compounds. These polythionates are reducing agents, in previous work, citric acid (Ferella et al., 2010) or the Fe(II) produced by an iron oxidizing bacteria (Xin et al.,2012a) was used as reducing agent to leach Mn (IV), in the present work the reducing agents were polythionates, so a possible reaction is Eq. (6). The percentages of metals extracted are shown in fig. 6; in two loads of fresh acid-reducing media, 100% of zinc is extracted while manganese reaches an efficiency of 65% .
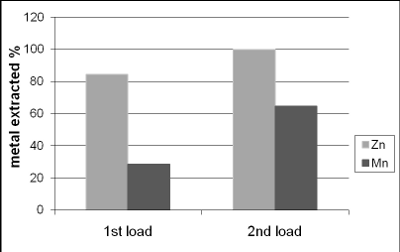
Figure 6. Concentration of Zn and Mn in the leaching reactor
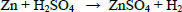 | (1) |
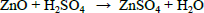 | (2) |
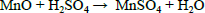 | (3) |
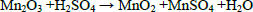 | (4) |
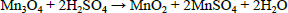 | (5) |
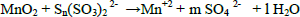 | (6) |
In the first load, pH reaches a stable value of 5 after 14 days of leaching, while in the second load the pH stabilizes at values below 2, meaning that there is an excess of acid in that step.
Comparison between commercial and biological acid media
Results from the analysis of samples from the S1 and S2 flasks indicate that the quantity of Mn extracted increases by a 60% in S2 (biological acid) in comparison with S1 (commercial acid), and for Zn, the increment of the extracted mass was 40%. These results show a better metal extraction performance for the biological acid than the commercial one. The extraction of Mn in S2 increases because of the presence of reducing compounds in the biological leaching medium (see Eq. 6).
After-leaching solid characterization
From EDS spectrum of the solid left after the acid leaching step, it can be observed that two major intensity peaks belong to Mn and O, which suggests that the principal component in the solid after the leaching step is a manganese oxide. No other metals are present in the spectrum in significant concentrations. This indicates that the bioleaching solid residue is one of the reusable products from the process. X-ray analysis of this solid calcined at 500deg;C shows the presence of Mn2O3. BET surface area was 7 m2 g-1.
C. Metal recovery from leachate
Electrolysis
During electrolysis experiments, metallic zinc was deposited in the cathode, Eq. (7), and a brown powder, which is a manganese oxide, in the anode. A change in the colour of the electrolytic bath from uncoloured to green was also observed; it could be a consequence of electrode corrosion, especially in the anode, which showed a mass loss at the end of the electrolysis.
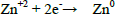 | (7) |
During manganese dioxide electrodeposition in the leaching acid solution, the reactions are eq. (8) for the anodic oxidation, and eq. (9) for the disproportionation reaction.
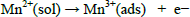 | (8) |
 | (9) |
The Mn2+ ions produced return to the solution leaving a cation vacancy on the deposit surface. In this oxidation, more H+ ions are formed, so acidity grows up in the electrolytic bath (Aldekani et al., 2007).
After 68 hours of electrolysis, 36% of manganese and 66% of zinc could be recovered from the leaching solution. The pH changed from 5 to 1.8. Low efficiency in the recovery of manganese could be a consequence of the regeneration of cation Mn2+ in the disproportionation reaction, Eq. (9), and the mass loss of the anodic deposit in the filtration step. Efficiency in the recovery of zinc could be affected by low pH (1.5) at the end of the electrolysis; metallic zinc can be dissolved in that acid medium.
Current efficiency for both deposits was less than 10%. This parameter can be lowered by H2 evolution caused by impurities in the solution; and by the low concentrations of both metals in the electrolytic bath (9000 ppm Zn and 4000 ppm Mn) (Souza and Tenorio, 2004).
Characterization of anodic and cathodic deposit
The X-Ray analysis evidenced the presence of Mn2O3 in the solid calcined at 500deg;C. BET surface areas of the sample was 442.4 m²/g, and for the sample calcined at 500°C, it was 44.0 m²/g. That difference in the areas could be a consequence of a change of phases by crystalization of the initial amorphous phase in the calcination step. Acid digestion and atomic absorption characterization of the deposits indicate 90% of purity for the cathodic deposit (metallic Zn) and 82% for the MnOx. The main impurity was Fe from the anodic corrosion. The SEM micrograph (Fig. 7) shows that the anodic deposit presents a laminar morphology.
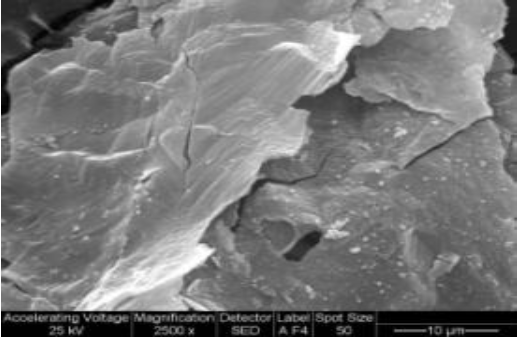
Figure 7. SEM micrograph of the anodic deposit.
III. CONCLUSIONS
The research demonstrated that biological sulphuric acid and polythionates allow the recovery of metals contained in alkaline and Zn-C batteries, with better efficiency than a commercial sulphuric acid of the same concentration (in particular for manganese), due to the presence of polythionates in the first one. The optimised operation conditions of the biological reactor determined in this work are: 8 kg sulphur mass (-3.5 and +5 mesh), 10 L mineral medium, 138 L/h air flow, end point of the batch culture 350 proton mM. This bioreactor was installed 5 years ago, and is still producing biological sulphuric acid and polythionates.
The biological production of sulphuric acid is under the cost of a commercial one and can be reduced even more (mineral medium composition optimisation, end point of the culture, design parameters of the reactor, incrementing available sulphur surface).
In the bioleaching reactor, extractions of 100% zinc and 65% manganese were reached and the manganese that was not leached remains as manganese oxide in the solid, one of the products of the process, so it is not necessary to extract all the manganese contained in the battery powder. Steel cases firstly separated from the battery powder can be treated with a pyrometallurgical process.
In the electrolysis of the leaching liquor, in one step at room temperature metallic Zn (90% purity) was obtained as the cathodic deposit, and a high surface manganese oxide as the anodic deposit (85% purity) The main contaminant of both deposits is Fe that comes from anodic corrosion. Low efficiency in this step may be caused by H2 evolution during electrolysis, anode corrosion, impurities, and low concentration of metals in the electrolytic bath. The electrolytic reactor efficiency can be improved further, for example, by using more concentrated leaching solutions and changing the electrodes material.
It was demonstrated in this work that metallic Zn and manganese dioxide can be recovered from spent alkaline and Zn-C batteries in a biotechnological pilot plant with the economic and ecological advantages of biological sulphuric acid and polythionate production. The recycling costs could be paid in part by selling the metals obtained.
REFERENCES
1. Adelkhani, H., M. Ghaemi and S.M. and Jafari, "Cycle life improvement of alkaline batteries via optimization of pulse current deposition of manganese dioxide under low bath temperatures," J. Power Sources, 163, 1091-1104 (2007).
2. Argentina, Argentinean National Law 26.184 (2006).
3. Bernardes, A.M., D.C.R. Espinosa and J.A.S. Tenorio, "Recycling of batteries: a review of current processes and technologies," J. Power Sources, 130, 291-298 (2004).
4. Bosio, V., M. Viera and E. Donati, "Integrated bacterial process for the treatment of a spent nickel catalyst," J. Hazard. Mater., 154, 804-810 (2008).
5. Brierley, J.A. and C.L. Brierley, "Present and future commercial applications of biohydrometallurgy," Hydrometallurgy, 59, 233-239 (2001).
6. Brierley, C.L., "Biohydrometallurgical prospects," Hydrometallurgy, 104, 324-328 (2010).
7. Bullock, J. and B. Kristiansen, Biotecnología Básica, Editorial Acribia, España (1995).
8. Cerruti, C., G. Curutchet and E. Donati, "Biodissolution of spent nickel-cadmium batteries using Thiobacillus ferrooxidans," J. Biotechnol., 62, 209-219, (1998).
9. Ceskova, P., M. Mandl, S. Helanovan and J. Kasparovska, J. "Kinetic studies on elemental sulfur oxidation by Acidithiobacillus ferrooxidans," Biotechnol. Bioeng., 68, 24-30 (2002).
10. Energizer, Energizer Carbon-Zinc Handbook, http://data.energizer.com/PDFs/carbonzinc_appman.pdf (2001).
11. Energizer, Energizer Alkaline Handbook, http://data.energizer.com/PDFs/alkaline_appman.pdf, (2008)
12. EPBA, http://www.epbaeurope.net (2006).
13. Espinosa, D.C.R. ,A.M. Bernardes and J.A.S. Tenório, "Brazilian policy on battery disposal and its practical effects on battery recycling," J. Power Sources, 137, 134-139 (2004).
14. European Communities, Directive 2006/66/EC of The European Parliament and of The Council On Batteries and Accumulators and Spent Batteries and Accumulators, Strasbourg, 6 September (2006).
15. European Communities, European Union Battery Extended Impact Assessment (2003).
16. Ferella, F., G. Furlani and M. Navarra, "Hydrometallurgical plant to recycle alkaline and Zn-C spent batteries: process and economic analysis," 2nd International Conference on Engineering for Waste Valorization, Patras, Greece (2008).
17. Ferella, F., I. De Michelis, F. Beolchini, V. Innocenzi and F. Veglio, "Extraction of zinc and manganese from alkaline and zinc-carbon spent batteries by citric-sulphuric acid solution," Int. J. Chem. Engineering, 1-13 (2010).
18. Konishi, Y., S. Asai and N. Yoshida, "Growth kinetics of Thiobacillus thiooxidans on the surface of elemental sulphur," Appl. Environ. Microbiol., 61, 3617-3622 (1995).
19. Morin, D., A. Lips, T. Pinches, J. Huisman, C. Frias, A. Norberg and E. Forssberg, "BioMinE - Integrated project for the development of biotechnology for metal-bearing materials in Europe," Hydrometallurgy, 83, 69-76 (2006).
20. Provazi, K., B. Amaral Campos, D.C.R. Espinosa and J.A.S. Tenório, "Metal separation from mixed types of batteries using selective precipitation and liquid-liquid extraction techniques," Waste Manage., 31, 59-64 (2011).
21. Rydh, C.J. and M. Karlstrom, "Life cycle inventory of recycling portable Nickel-cadmium batteries," Resour. conserv. recy., 34, 289-309 (2002).
22. Sayilgan, E., T. Kukrer, G. Civelekoglu, F. Ferella, A. Akcil, F. Veglio and M. Kitis, "A review of technologies for the recovery of metals from spent alkaline and zinc-carbon batteries," Hydrometallurgy, 97, 158-166 (2009a).
23. Sayilgan, E., T. Kukrer, F. Ferella, A. Akcil, F. Veglio and M. Kitis, "Reductive leaching of manganese and zinc from spent alkaline and zinc-carbon batteries in acidic media," Hydrometallurgy, 97, 73-79 (2009b).
24. Shrihari, R., J. Bhavaraju, M. Kodak, R. Kumar and K.S. Gandhi, "Dissolution of sulphur particles by Thiobacillus ferrooxidans: substrate for unattached cells," Biotechnol. Bioeng., 41, 612-616 (1993).
25. Sousa, C.C.B.M. and J.A.S. Tenório, "Simultaneous recovery of zinc and manganese dioxide from household alkaline batteries through hydrometalurgical processing," J. Power Sources, 136, 191-196, (2004).
26. Toro, L., F. Veglio, F. Beolchini, F. Pagnanelli, M. Zanetti and G. Furlani, "Process and plant for the treatment of run-down batteries," Eur. Pat. Appl., EPXXDW EP 1684369 A1 20060726) (2006).
27. Xiang, Y., P. Wu, N. Zhu, T. Zhang, W. Liu, J.Wu and and L. Ping, "Bioleaching of copper from waste printed circuit boards by bacterial consortium enriched from acid mine drainage," J. Hazard. Mater. 184, 812-818 (2010).
28. Xin, B., D. Zhang, X. Zhang, Y. Xia, F. Wu, B. Chen and L. Li,"Bioleaching mechanism of Co and Li from spent lithium-ion battery by the mixed culture of acidophilic sulfur-oxidizing and iron-oxidizing bacteria," Bioresour. Technol., 100, 6163-6169, (2009).
29. Xin, B., W. Jiang, H. Aslam, K. Zhang, Ch. Liu, R. Wang and Y. Wang, "Bioleaching of zinc and manganese from spent Zn-Mn batteries and mechanism exploration," Bioresour. Technol., 106, 147-153 (2012a).
30. Xin, B., W. Jiang, X. Li, K. Zhang, C. Liu, R. Wang and Y. Wang, "Analysis of reasons for decline of bioleaching efficiency of spent Zn-Mn batteries at high pulp densities and exploration measure for improving performance," Bioresour. Technol., 112, 186-192 (2012b).
31. Zhao, L., N. Zhu and X. Wang, "Comparison of bio-dissolution of spent Ni-Cd batteries by sewage sludge using ferrous ions and elemental sulfur as substrate," Chemosphere, 70, 974-981 (2008).
Received: March 3, 2013
Accepted: August 13, 2013
Recommended by Subject Editor: María Luján Ferreira












