Servicios Personalizados
Articulo
Latin American applied research
versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.44 no.2 Bahía Blanca abr. 2014
Ultrasound-assisted extraction of total flavonoids from leaves of Syringa Oblata Lindl.
Z. Sheng†‡ - Y. Wang‡ - P. Wan‡ and Y. Li‡
† College of Veterinary Medicine, Heilongjiang Bayi Agricultural Univeristy, Daqing 163319, P.R. China. shengzunlai@yahoo.com
‡ College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, P.R. China shengzunlai@neau.edu.cn
Abstract— Leaves of Syringa Oblata Lindl. possess some important biological activities, such as antibacterial, antiviral, anti-inflammatory and antioxidant activities. These biological properties are mainly attributed to total flavonoids content. In this paper, ultrasound-assisted extraction of total flavonoids from leaves of Syringa Oblata Lindl. was studied. Effects of several experimental parameters, such as concentration of extracting solvent, ratio of liquid to material, extraction temperature, and time of sonication on extraction efficiencies of total flavonoids were evaluated. The best extraction conditions were: 1 g plant sample with 20 mL of 50% ethanol, at 60°C for 50 min, obtaining a yield of total flavonoids of 92.00 ± 0.87 mg/g of plant. The results indicated that high amounts of total flavonoids can be extracted from leaves of Syringa Oblata Lindl. by ultrasound-assisted extraction technology.
Keywords— Ultrasound-assisted Extraction; Total Flavonoids; Leaves of Syringa Oblata Lindl.
I. INTRODUCTION
Syringa Oblata Lindl is an economic plant, which is distributed throughout China, Korea, Japan and many European nations. The leaves of Syringa Oblata Lindl have been extensively used in China for antibacterial, antiviral, anti-inflammatory and anti-diarrhea due to high content of flavonoids (El-Desouky and Gamal-Eldeen, 2009; Nenadis et al., 2007; Wang et al., 2008a). Therefore, the content of total flavonoids is critical to the quality control of the leaves of Syringa Oblata Lindl.. Although it is widely distributed as wild and cultivated species in China, the leaves of Syringa Oblata Lindl remain an underutilized natural resource so far. For the sake of making better use of the resource abroad, more research is immediately required for efficient procedures of the leaves extracts.
The extraction of active ingredients from plants can be carried out in a variety of ways, such as maceration extraction, reflux extraction, Soxhlet extraction and supercritical fluid extraction (Cannon and Fenske, 1936; Johansen et al., 1996; Sultana et al., 2009). The extraction efficiency is usually low using conventional methods, such as maceration extraction and Soxhlet extraction. However, the drawback could be greatly improved using ultrasound-assisted extraction. Owing to acoustic cavitation, the ultrasound-assisted extraction (UAE) allows the solvent to penetrate cell walls and promotes the release of soluble compounds from the plant body (Eh and Teoh, 2012; Konwarh et al., 2012). Therefore, the ultrasound is capable of accelerating the extraction efficiency. Ultrasound-assisted extraction is now recognized as an efficient extraction technique that dramatically shortens extraction time, increasing yields and lowering energy input. In the foretime, this extraction technique was chiefly exploited on the laboratory scales, however, it has already found industrial applications in this field now (Sivakumar et al., 2009; Vilkhu et al., 2008; Zhang et al., 2008). The new technology attracts much more attention in the department of extraction and separation in recent years.
Currently, new techniques such as microwave-assisted extraction (Moreira et al., 2012; Sousa et al., 2010) and supercritical CO2 extraction (Liu et al., 2011; Wang et al., 2008b) have been employed to optimize the extraction process. However, compared with those techniques, the use of UAE is more convenient and affordable. While many papers have been published dealing with the UAE of different plant materials (Eh and Teoh, 2012; Konwarh et al., 2012), its effect on the UAE of this important herb, leaves of Syringa Oblata Lindl, has not been reported. In order to improve total flavonoids extraction efficiency by UAE, the effects of operating parameters on the release of flavonoids were investigated in this study.
II. MATERIALS AND METHODS
A. Apparatus
For the ultrasound-assisted extraction experiments, an ultrasonic bath was used as an ultrasound source. The bath (KQ-100DB, Kunshan Ultrasound Co. Ltd., China) was a rectangular container (23.5×13.3×10.2 cm), to which 50 kHz transducers were annealed at the bottom. The bath power rating was 100 W on the scale of 0-10. Water in the ultrasonic bath was circulated and regulated at constant desired temperature to avoid the temperature rise caused by ultrasonic exposure. UV-1700 spectrophotometer (Shimadzu Corporation, Japan) was used for total flavonoids analysis of sample, RE-52AA rotavapour (Shanghai Yarong Biochemistry Instrument Factory, Shanghai, China) was used for concentration of sample.
B. Reagents and materials
Standard of rutin was provided by the Chinese Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Ethanol, Sodium nitrite, Aluminum nitrate and Sodium hydroxide were of analytical grade and purchased from Hangzhou Reagent Company (Hangzhou, PR China). Deionized water was purified by a Milli-Q academic water purification system (Millipore, Bedford, MA, USA). The 0.45 μm microporous membrane was made of nylon 6, which was purchased from Tianjin Jinteng Experiment Equipment Co., Ltd.
Leaves of Syringa Oblata Lindl were collected from Heilongjiang province (China) and authenticated by Prof. Yanbing Li of Heilongjiang University of Traditional Chinese Medicine, China.
C. Extraction method
Leaves of Syringa Oblata Lindl were ground into power (particle diameter: 0.2-0.5 mm) in a blender. 5 g samples were mixed with ethanol solvent in designed conditions, placed in ultrasonic cleaning bath having as coupling liquid water. The extraction solutions were filtered off through 0.45 μm microporous membrane. The filtrate was collected and diluted to 100 mL with deionized water for determination of flavonoids.
D. Determination of total flavonoids content
The total flavonoids content in the extracts was determined using aluminum chloride colorimetric method (Sheng et al., 2013) using rutin as a standard. The rutin solutions were prepared at 8.16, 16.32, 24.48, 32.64, 40.8 and 48.96 mg/mL in 80% ethanol (v/v). For the analysis, 1 mL of the rutin solutions, 5 mL of 80% (v/v) ethanol and 1 mL of 5% (w/w) NaNO2 were mixed for 6 min, and then 1 mL of 10% AlCl3 (w/w) was added and mixed, 6 min later, 10 mL of 1 mol/L NaOH was added. With 15 min standing, the absorbance of the mixture was then measured using a spectrophotometer at 510 nm (Li and Mao, 2011). For the blank sample, rutin solution in the mixture solutions was substituted by the same volume of deionized water. In this designed study, the absorbance of the mixture was performed with concentration of rutin as a standard curve. The calibration curve (y= -0.0184+0.01251x, where y is absorbance value of mixture, x is rutin concentration) ranged 8.16-48.96 mg/mL (R2=0.9993). The absorbance values of the extracted samples solutions were also obtained using aluminum chloride colorimetric method. The total flavonoids content in the extracts was expressed as rutin equivalents (μg rutin/g sample) through the calibration curve of rutin.
All the experiments were conducted in triplicate, and the average values ± SD (standard deviation) were reported.
E. Statistical analyses of data
Statistical analysis of the single-factor experimental data was performed with SAS 9.1 software (SAS Institute Inc., Cary, NC, USA). The Dunnett's one-tailed t-tests permitted the checking of the statistical significance of the means in the different levels of parameters.
III. RESULTS AND DISCUSSION
A. Ethanol concentration selection
The selection of ethanol concentration is very important when extracting multi-residues with UAE method. To investigate the effect of ethanol concentration on extraction of flavonoids, a range from 0 to 100% of ethanol concentration had been used for investigation. As shown in Fig. 1, the extraction amount of flavonoids was greatly influenced by the concentration of ethanol. The extraction yield initially increased with the increase of ethanol concentration until reached a maximum (88.24 ± 1.31mg/g) at 50%, after which it decreased. This should be due to the differences in polarity between the solvent and the total flavonoids in this herb. The polarity of flavonoids is much lower than water and much higher than ethanol, so that it is not well solubilized in 0% ethanol and 100% ethanol.
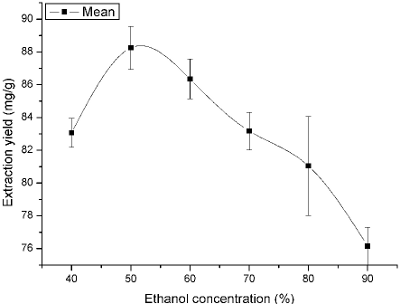
Fig. 1. Effect of ethanol concentration on the extraction yield.
Table 1 showed that significant differences existed among 40%, 70%, 80%, 90% and 50% (P < 0.05). It also indicated that the maximum extraction yield was gained by 50% ethanol (difference between means were negative). The negative values of 95% of upper confidence limits further confirmed that 50% ethanol should be adopted in this work.
Table1. The results of multiple comparisons of different ethanol concentration
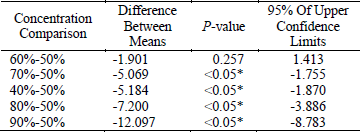
* The mean difference is significant at the 0.05 level
B. Effect of extraction temperature
Many physical properties of solvent, such as viscosity, diffusivity, solubility, vapor pressure and surface tension, were influenced by temperature. Therefore, the choice of the extraction temperature was another important step. In this study, effect of different temperature (20, 30, 40, 50, 60, and 70 °C) on the yield of flavonoids was investigated. The results were displayed in Fig. 2. The yield increased greatly when the temperature increased from 20 to 60 °C, and the highest yield was obtained at 60 °C. This enhanced yield of flavonoids with the increase of temperature might result from the increased diffusivity of the solvent into cells and enhanced desorption and solubility of flavonoids from the cells (Bimakr et al., 2011; Prommuak et al., 2008). However, the main effect of ultrasounds was cavitational bubbles collapse. The observed effects appeared due to acoustic cavitation, which was supported by the fact that a higher efficiency was observed at 60 °C rather than 70 °C. At 60 °C, the number of cavitation bubbles increased and their collapse was powerful enough to act on extraction. At 70 °C, although the number of bubbles was higher, their collapse was less efficient. It could be due to that at high vapor pressure, more bubbles were created but they collapsed with lower intensity owing to the smaller internal/external pressure difference (Boonkird et al., 2008; Hemwimol et al., 2006). This could be explained that there was a slight decrease in the yield when the temperature increased from 60 to 70 °C. Table 2 also represented there were significant differences among 20, 30, 40, 50, 70 and 60 °C (P < 0.05). It also indicated that the maximum extraction yield was gained at 60 °C (difference between means were negative). The negative values of 95% of upper confidence limits further confirmed that 60 °C should be ado adopted in this work.
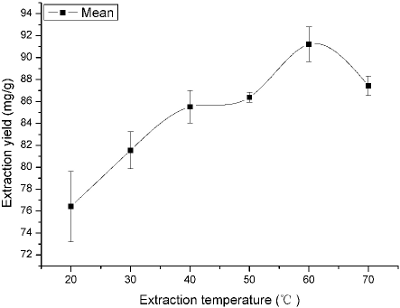
Fig. 2. Effect of extraction temperature on the extraction yield.
Table2. The results of multiple comparisons of different extraction temperature
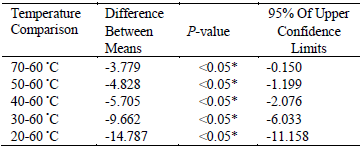
* The mean difference is significant at the 0.05 level
C. Effect of extraction time
Extraction time is not constant during the extraction stages. In this study, extraction time was, respectively, set at 10, 20, 30, 40, 50 and 60 min to examine the influence of extraction time on the yield of the flavonoids. As shown in Fig. 3, the yield of flavonoids reached a maximum percentage of 91.21 ± 1.58 when the extraction time was 60 min. After this point, the flavonoids yield started to maintain a descending dynamic equilibrium with increasing the extraction time. Compared with reflux extraction (Gulbostan and Watengul, 2011), UAE was found to enhance the extraction efficiency. This was due to the cavitational effects, which could accelerate swelling and disrupt the cell wall; it resulted in the intensification of mass transfer of solute constituents from the plant materials to solvent. The disruption of plant cells by micro-jets after the cavitation bubble collapsed could cause tissue disruption and a good penetration of the solvent into the tissue matrix (Sivakumar et al., 2009; Xia et al., 2011). Table 3 showed significant differences among 10, 20, 30, 40, 60 and 50 min (P < 0.05). It also indicated that the maximum extraction yield was gained at 50 min (difference between means were negative). The negative values of 95% of upper confidence limits further confirmed that extraction time of 50 min should be adopted in this work.
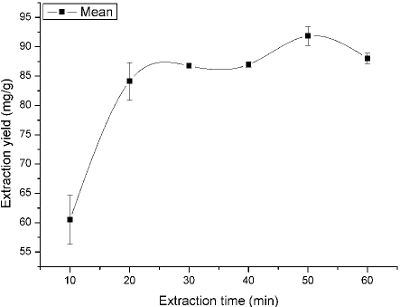
Fig.3. Effect of ultrasonic time on the extraction yield.
Table3. The results of multiple comparisons of different extraction time
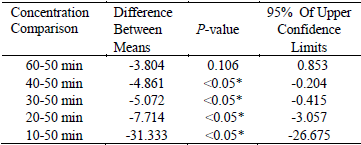
* The mean difference is significant at the 0.05 level
D. Effect of ratio of liquid to material
The yield of flavonoids was greatly influenced by different ratios of liquid to material (Luo and Chen, 2010). In general, extraction yield will increase if ratio of liquid to material increasing. However, it will result in a high process cost if ratio of liquid to material is too big. Therefore, proper ratio of liquid to material should be opted. In the present studies, ratio of liquid to material as an important extraction parameter was set at 10, 15, 20, 25 and 30 to investigate the influence of different ratios of liquid to material on the yield of the flavonoids.
As described in Fig. 4, the extraction yield increased as ratio of liquid to material increased from 10 to 20, and then it remained constant until 30. It could be explained that a large solvent volume increased the amount of aerosol and dissolved the constituents more electively, and both of which could lead to an enhancement of the extraction yield. Table 4 also represented that there were significant differences among 10, 15 and 20 (P<0.05), but no significant difference existed among 25, 30 and 20 (P>0.05). In order to reduce the solvent requirement and without compromising on the responses, ratio of liquid to material of 20 was selected in this work.
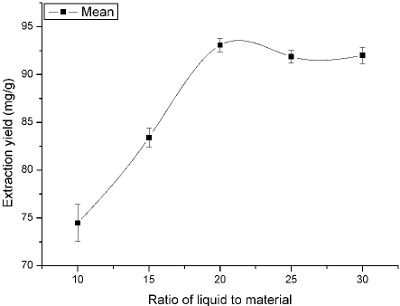
Fig.4. Effect of ratio of liquid to raw material on the extraction yield.
Table4. The results of multiple comparisons of different ratio of liquid to material
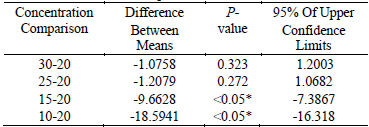
* The mean difference is significant at the 0.05 level
Under the above optimal conditions, the extraction rate of total flavonoids using ultrasound-assisted extraction was 93.07 ± 0.71. Although the extraction time of total flavonoids from the UAE pilot scale was longer than that from MAE pilot scale (Li and Mao, 2011), the yield of total flavonoids from the UAE pilot scale was greatly improved. In addition, the yield of total flavonoids from the UAE pilot scale was similar to that from industrial scale hot maceration (Gulbostan and Watengul, 2011). However, UAE shortened the extraction time and lowered the operating temperature, which resulted in considerably lower operating costs. This was certainly one of the greater advantages of UAE.
IV. CONCLUSIONS
An UAE method has been developed for the extraction of total flavonoids from the Leaves of Syringa Oblata Lindl. . Ultrasonic wave was a powerful tool, which efficiently improved the extracting performance of total flavonoids. Effects of several experimental parameters on the extraction yields of total flavonoids have been evaluated, and the optimal extraction conditions were 1 g plant sample with 20 ml of 50% ethanol and the extraction for 50 min at 60 °C under ultrasonic irradiation. Under the optimal conditions, the yield of total flavonoids was 93.07 ± 0.71 mg/g. The results obtained are helpful for the full utilization of Leaves of Syringa Oblata Lindl., which also indicated that the UAE is a powerful tool for the extraction of important phytochemicals from plant materials.
ACKNOWLEDGEMENTS
This research was supported by Heilongjiang University Animal Medical Key Laboratory (AMKL2012008).
REFERENCES
1. Bimakr, M., R.A. Rahman and F.S. Taip, "Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves," Food and Bioproducts Processing, 89, 67-72 (2011).
2. Boonkird, S., C. Phisalaphong and M. Phisalaphong, "Ultrasound-assisted extraction of capsaicinoids from Capsicum frutescens on a lab- and pilot-plant scale," Ultrasonics Sonochemistry, 15, 1075-1079 (2008).
3. Cannon, M. and M. Fenske, "Lubricating oils solvent extraction in a reflux extraction unit," Ind. Eng. Chem. Res, 28, 1035-1037 (1936).
4. Eh, A.L.S. and S.G. Teoh, "Novel modified ultra-sonication technique for the extraction of lycopene from tomatoes," Ultrasonics Sonochemistry, 19, 151-159 (2012).
5. El-Desouky, S.K. and A.M. Gamal-Eldeen, "Cytotoxic and anti-inflammatory activities of some constitu-ents from the floral buds of Syringa patula," Pharmaceutical Biology, 47, 872-877 (2009).
6. Gulbostan, A. and K. Watengul, "Extraction and deter-mination of total flavonoids in lilac," China Brewing, 8, 32-34 (2011).
7. Hemwimol, S., P. Pavasant and A. Shotipruk, "Ultra-sound-assisted extraction of anthraquinones from roots of Morinda citrifolia," Ultrasonics Sonochemistry, 13, 543-548 (2006).
8. Johansen, H.N., V. Glitsø and K.E.B. Knudsen, "Influence of extraction solvent and temperature on the quantitative determination of oligosaccharides from plant materials by high-performance liquid chromatography," Journal of Agricultural and Food Chemistry, 44, 1470-1474 (1996).
9. Konwarh, R., S. Pramanik and D. Kalita, "Ultra-sonication - A complementary 'green chemistry' tool to biocatalysis: A laboratory-scale study of lycopene extraction," Ultrasonics Sonochemistry, 19, 292-299 (2012).
10. Li, N. and Y.Q. Mao, "Microwave-Assisted Extraction of Flavonoids from the Leaves of Syringa oblate," Advanced Materials Research, 236, 309-312 (2011).
11. Liu, J., S. Lin and Z. Wang, "Supercritical fluid extraction of flavonoids from Maydis stigma and its nitrite-scavenging ability," Food and Bioproducts Processing, 89, 333-339 (2011).
12. Luo, C. and Y.S. Chen "Optimization of extraction technology of Se-enriched Hericium erinaceum polysaccharides by Box-Behnken statistical design and its inhibition against metal elements loss in skull," Carbohydrate Polymers, 82, 854-860 (2010).
13. Moreira, M.M., S. Morais and A.A. Barros, "A novel application of microwave-assisted extraction of polyphenols from brewer's spent grain with HPLC-DAD-MS analysis," Analytical and Bioanalytical Chemistry, 403, 1019-1029 (2012).
14. Nenadis, N., J. Vervoort and S. Boeren, "Syringa oblata Lindl var. alba as a source of oleuropein and related compounds," Journal of the Science of Food and Agriculture, 87, 160-166 (2007).
15. Prommuak, C., W. De-Eknamkul and A. Shotipruk, "Extraction of flavonoids and carotenoids from Thai silk waste and antioxidant activity of extracts," Separation and Purification Technology, 62, 444-448 (2008).
16. Sheng, Z.L., P.F. Wan and C.L. Dong, "Optimization of total flavonoids content extracted from Flos Populi using response surface methodology," Industrial Crops and Products, 43, 778-786 (2013).
17. Sivakumar, V., J.L. Anna and J. Vijayeeswarri, "Ultra-sound assisted enhancement in natural dye extraction from beetroot for industrial applications and natural dyeing of leather," Ultrasonics Sonochemistry, 16, 782-789 (2009).
18. Sousa, A.M., V.D. Alves and S. Morais, "Agar extraction from integrated multitrophic aquacultur-ed Gracilaria vermiculophylla: evaluation of a microwave-assisted process using response surface methodology," Bioresource Technology, 101, 3258-3267 (2010).
19. Sultana, B., F. Anwar and M. Ashraf, "Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts," Molecules, 14, 2167-2180 (2009).
20. Vilkhu, K., R. Mawson and L. Simons, "Applications and opportunities for ultrasound assisted extraction in the food industry-A review," Innovative Food Science and Emerging Technologies, 9, 161-169 (2008).
21. Wang, J., J. Yin and Z. Liu, "Microscopic identification and quantitative determination of four species of Folium syringae," Asian Journal of Traditional Medicines, 3, 230-237 (2008).
22. Wang, L., B. Yang and X. Du, "Optimisation of supercritical fluid extraction of flavonoids from Pueraria lobata," Food Chemistry, 108, 737-741 (2008).
23. Xia, E.Q., X.X. Ai and S.Y. Zang, "Ultrasound-assisted extraction of phillyrin from Forsythia suspensa," Ultrasonics Sonochemistry, 18, 549-552 (2011).
24. Zhang, H., T. Yang and Z. Li, "Simultaneous extraction of epimedin A, B, C and icariin from Herba Epimedii by ultrasonic technique," Ultrasonics Sonochemistry, 15, 376-385 (2008).
Received: January 16, 2013
Accepted: September 10, 2013
Recommended by Subject Editor: María Luján Ferreira












