Servicios Personalizados
Articulo
Latin American applied research
versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.44 no.2 Bahía Blanca abr. 2014
Study on structure-activity relationship of 2E-3-phenyl propenyl acyloxy alkyl phosphonate molecular derivatives
H.-M. Bi† - P.-T. Xie† - J.-P. Hu† - Y. Liu† - F.-Y. You† and L.-P. Meng‡
† Handan key laboratory of organic small molecule materials, Handan College, Hebei Handan 056002, China. binbi99@163.com
‡ Hebei Normal University, Shijiazhuang, Hebei Province 050091, China
Abstract— The quantum chemistry calculation of 2E-3-phenyl propenyl acyloxy alkyl phosphonate derivates was carried out to investigate the relationship between the structure and plant regulator activity of these compounds. All the compounds were studied by HF method with 6-31G* basis set using the PCM model within the self-consistent reaction field method to assess solvent effects, and then we established mathematical correlation between the properties and bioactivity of these compounds. The result showed that the bioactivity of these compounds has a linear relationship with the frontier orbital energy and other properties. At the same time, the active sites of these molecules were predicted. These compounds are electron acceptors.
Keywords— Phosphonate Derivatives; Quantum Chemistry; Quantitative Structure-activity Relationships; Solvent Effects.
I. INTRODUCTION
Phosphonate derivatives are important insecticides (Bairiki et al., 2012; Heinze et al., 2012; Chang et al., 2011), have a wide activity about herbicidal sterilization and plant growth regulating and so on (Shaekhov et al., 2011; He, 2003). Wang Tao synthesized 2E-3-phenyl propenyl acyloxy alkyl phosphonate molecular derivatives (Wang et al., 2011; He and Liu, 2001) and determined the biological activity of these compounds. The results showed that these compounds have good plant regulating activity to the plant root cells under certain conditions.
A theoretical study of 2E-3-phenyl propenyl acyloxy alkyl phosphonate derivatives was carried out. The study found a correlation between the antibacterial activity of these compounds (Bittner et al., 2009; Gacitúa et al., 2009) and structural parameters. The main factors affecting the biological activity were analyzed, the influence in biological activity from the changes in the molecular structure was explained and the mechanism and sites of action of compounds were discussed.
II. METHODS
A. Method of Calculations
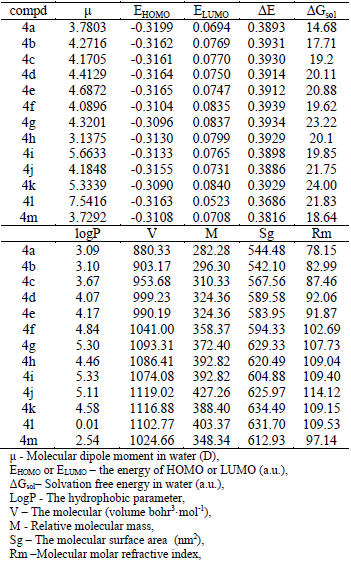
All the compounds were studied by HF method with 6-31G* basis set using the PCM model within the self-consistent reaction field method to assess solvent effects. For these molecules, the solvation free energies (ΔGsol) in water and the dipole moments in water were obtained. Harmonic vibrational frequencies calculated at the same level were used for the characterization of stationary points as a minimum. All quantum calculations were performed with the Gaussian 03 program. The logP, V, M, Sg and Rm were calculated by Hyperchem using the optimized configuration from the result of Gaussian 03.
B. Results and discussions
Stability configurations and natural charge
Figure 1 depicts the structure of compounds. The atomic natural charges of compounds are given in Table 1. These data show that the negative charge is mainly concentrated in the C (8), and O (10) of carbonyl, These atoms make the electronegative area of the compound and they could combine with positive area of receptor. The positive charge is mainly concentrated in the C (9) of carbonyl , P, and N of R in the compound. These atoms are the positive area of the molecule, and they could combine with negative area of receptor.
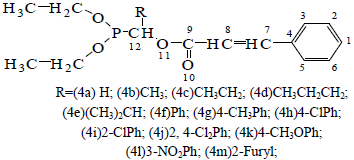
Figure 1. The structure of compounds
Table 1 -The atomic natural charge of compounds
The energy, main composition and proportion of the frontier molecules orbital
According to the theory of molecular orbital (MO), the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) have the greatest influence on the activity of compounds. The reaction between active molecule and macromolecular receptor operated on the frontier molecules orbital. EHOMO is the energy of HOMO, which relates to the molecular electron donor abilities. ELUMO is the energy of LUMO, which relates to the molecular ability of electron acceptance. For pesticide molecules, too low-ELUMO or too high-EHOMO means that the intrinsic molecular activity is too strong and it is easy to be metabolized in organism, The effect of pesticides is difficult to control, so the ELUMO or EHOMO of a pesticide molecule should be suitable to estimate an expected activity value (Wei et al., 2009; Yang et al., 1998).
From Table 2, when the receptor is the root cells of a monocotyledon (heat), the data of promoted activity would be the best for compound 4l. The ELUMO of 4l is the lowest, so it has strong ability to accept electrons. This may be the reason that these compounds promote growth for the plant root. The data of promoted activity would be bad for compound 4k, where the ELUMO is high. The ELUMO of compounds 4e and 4m is comparatively low, and the data of promoted activity of them would be better than the one of 4k. We can conclude that the lower ELUMO of compound, the better the relative promoted activity of the compound is. The conclusion is consistent with the experimental values.
Table 2. The energy of the molecular frontier orbitals (eV)
From the above discussion, the mechanism of action of 2E-3-phenyl propenyl acyloxy alkyl phosphonate molecular derivatives on the root cells of monocotyledon (wheat) is mediated by the ELUMO, the main factor affecting activity. When these compounds react with the receptors, they accept electrons.
From Table 3, the main composition and proportion of ELUMO of 4l are in the R-C, R-N, and P, and for other compounds are in the C (1) − C (9) and O (10), the positive charge of them are mainly concentrated in the C (9), and the charge of C (1) − C (8) are negative. The electronic affinity has a major influence on the biological activity. Some atoms could accept electrons from receptor. The main composition and proportion of EHOMO of these compounds are in the C (1) − C (8) and O (10), the negative charge is mainly concentrated in these atoms and they have strong ability to provide electrons.
Table 3. The main composition and proportion of molecular frontiers orbital
The main composition and proportion of ELUMO of 4l is in the R-C, R-N, and P; the positive charge is mainly concentrated in this area. These atoms could accept electrons. The nitrobenzene group plays an important role because of the strong electron-withdrawing effects. So, when the receptor is the root cells of monocotyledon, the growth of root cells will be promoted because the phosphonate group accepts electrons from target cells. The analysis of QSAR.
The parameters
The results of quantum calculation are included in Table 4.
Table 4-Results of Calculation-Molecular Parameters
Correlation analysis
The SPSS statistical software was used to correlation analysis. The independent variables are the parameters in Table 4, and the dependent variable was the rate of inhibition. The correlation coefficients are presented in Table 5.
Table 5-The correlation coefficients between the parameters and the rate of inhibition
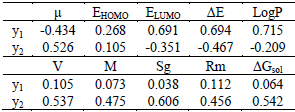
From Table 5, when the receptor is the root cells of monocotyledon, the y1 was significantly associated with ELUMO, ΔE and logP; when the receptor is the root cells of dicotyledon, the y2 was significantly associated with V, Sg and ΔGsol.
Regression analysis
The QSAR results of 2E-3-phenyl propenyl acyloxy alkyl phosphonate molecular derivatives were analyzed. The higher correlation parameters of table 5 were selected as independent variables and activity data as the dependent variable (y) to perform multiple linear regression analysis.
The ELUMO and ΔE were selected as independent variables, and y1 as the dependent variable. The curve fitting to model (1) resulted as follows:
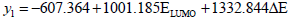 | (1) |
with n=13; R=0.707; Se=0.913; F=4.989; Q=0.774, where
n - The number of samples in the model
R - Multiple correlation coefficient
Se - Standard deviation
F - Sher's statistics
Q - Quality factor (Q=R/Se)
The value of ELUMO was proportional to the inhibition ratio of root cells of monocotyledon, which is consistent with the previous discussion.
The y2 was significantly associated with V, Sg and ΔGsol, but the ΔGsol was associated with V and Sg. The ΔGsol was excluded because of their low association with y2. V and Sg were selected as independent variables, and y2 as the dependent variable being the curve fitting to model (2) as follows:
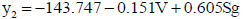 | (2) |
n=13; R=0.646; Se=0.913; F=3.589; Q=0.708
It could be concluded from model (2) that V shows the differences of the size of R, and the parameter Sg was the molecular factor to influence the activity of compounds. These results give clues for further molecular design.
III. CONCLUSIONS
(1) When the receptor is the root cells of monocotyledon, the ELUMO of phosphonates is the main factor to influence activities of these kinds of compounds.
(2) The results indicate that C (9) of carbonyl and nitrobenzene group is the important active site for monocotyledon root cells interactions. They promote growth.
(3) The influence of molecular volume and surface area can not be ignored for the active site of dicotyledon.
ACKNOWLEDGEMENTS
This project was supported by the Science and technology projects of Hebei Province (Contract No. 10273939).
REFERENCES
1. Bayrakci, M., Ş. Ertul and M. Yilmaz, "Synthesis of new water-soluble phosphonate calixazacrowns and their use as drug solubilizing agents," Journal of Inclusion Phenomena and Macrocyclic Chemistry, 74, 293-303 (2012).
2. Bittner, M., M.A. Aguilera, V. Hernández, C. Arbert, J. Becerra and M.E. Casanueva, "Fungistatic activity of essential oils extracted from Peumus boldus Mol., Laureliopsis philippiana (Looser) Schodde and Laurelia sempervirens (Ruiz & Pav.) Tul. (Chilean Monimiaceae)," Chilean Journal of Agricultural Research, 69, 30-37 (2009).
3. Chang, S.C., B. Condon, E. Graves, M. Uchimiya, C. Fortier, M. Easson and P. Wakelyn, "Flame retardant properties of triazine phosphonates derivative with cotton fabric," Fibers and Polymers, 12, 334-339 (2011).
4. Gacitúa, S.A., C.F. Valiente, K.P. Díaz, J.C. Hernández, M.M. Uribe and E.V. Sanfuentes, "Identification and Biological Characterization of Isolates with Activity Inhibitive Against Macrophomina phaseolina (Tassi) Goid," Chilean Journal of Agricultural Research, 69, 526-533 (2009).
5. He, H.W., "Phosphorus chemistry, A field full of vitality and scientific opportunities," Journal of Organometallic Chemistry, 23, 155-161 (2003).
6. He, H.W. and Z.J. Liu, " progresses in Research of oxophosphonic Acid Derivatives with Herbicidal Activity," Chinese Journal of Organic Chemistry, 21, 878-883 (2001).
7. Heinze, T., V. Sarbova, M.C. Vieira Nagel, "Simple synthesis of mixed cellulose acylate phosphonates applying n-propyl phosphonic acid anhydride," Cellulose, 19, 523-531 (2012).
8. Shaekhov, T.R., E.M. Gibadullina, K. Voronina Yu, V.V. Syakaev, D.R. Sharafutdinova, A.R. Burilov and M.A. Pudovik, "Synthesis of novel phosphorus-containing sterically hindered phenols by the reaction of diphenyl (3,5-di-tert-butyl-4-oxocyclohexa-2,5-dienylidene) methylphosphonate with phenols," Russian Chemical Bulletin, 60, 1999-2002 (2011).
9. Wang, T., H.J. Huang, J. Luo and S.T. Zhou, "Synthesis and Biological Activity of O,O-Diethyl-(2E-3-Phenacryloyloxy) Alkyl Phosphonates," Journal of JiangXi Normal University (Natural Sciences Edition), 35, 15-20 (2011).
10. Wei, T.B., Y.L. Leng, Y.C. Wang, J.H. Zhang and Y.M. Zhang, "Biology Activity and Quantum Chemical Calculation of p-Toluene Sulfonylamido Acetacylhydrazone Derivatives," Chinese Journal of Organic Chemistry, 29, 216-221 (2009).
11. Yang, G.F., H.Y. Liu, Y.H. Zheng and X.F. Yang, "Design, Syntheses and Biological Activity of Novel Herbicides Targeted ALS () Studies on The Crystal Structure, Quantum Chemistry and Structure-Activity Relationship of 1,2,4-Triazolo[1,5-a] Pyrimidine-2-sulfonamides Compounds," Acta Chimica Sinica, 56, 729-735 (1998).
Received: December 15, 2012
Accepted: August 18, 2013
Recommended by Subject Editor: María Luján Ferreira












