Servicios Personalizados
Articulo
Latin American applied research
versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.45 no.1 Bahía Blanca ene. 2015
Photocatalytic activity of ZnAl2O4 spinel for procion red degradation under UV irradiation
F. M. Stringhini†, M. A. Mazutti†, G. L. Dotto†, S. L. Jahn†, A. Cancelier†, O. Chiavone-filho‡ and E. L. Foletto†,*
† Chemical Engineering Department, Federal University of Santa Maria, 97105-900, Santa Maria, Brazil
‡ Chemical Engineering Department, Federal University of Rio Grande do Norte, 59072-970, Natal, Brazil
* E-mail: efoletto@gmail.com
Abstract— In this work, ZnAl2O4 spinel was used as photocatalyst for dye degradation in aqueous solutions under UV irradiation. This material was prepared by a route with chitosan as template. The effects of process variables, such as, initial dye concentration and photocatalyst concentration were investigated. Kinetic parameters were obtained and the reaction order was determined. The results revealed that both studied variables presented strong influence on the dye degradation efficiency, which attained 100% in some cases. It was found that the dye photocatalytic degradation under UV irradiation followed a first order kinetic behavior. The results indicated that ZnAl2O4 spinel can be used to treat dye containing effluents.
Keywords— Zinc Aluminate; ZnAl2O4; Photocatalysis; Dye; UV Irradiation; Kinetics.
I. INTRODUCTION
Photocatalysis has been widely used for the destruction of organic pollutants from aqueous solutions (Morales et al., 2012; Collazzo et al., 2012a). Different materials have been tested as photocatalysts, such as, TiO2 (Alfano and Cassano, 2009; Krishnan and Swaminathan, 2010; Silva et al., 2012), ZnO (Cataño et al., 2012; Silva et al., 2013), Nb2O5 (Collazzo et al., 2012b), CdS fibers (Hernández-Gordillo et al., 2013), Mg-Zn-Al layered double hydroxides (Valente et al., 2009) and Zn2SnO4 (Foletto et al., 2013). However, the use of ZnAl2O4 as photocatalyst agent in degradation reactions of organic pollutants from aqueous solution has been little investigated.
Zinc aluminate (ZnAl2O4) is a ternary oxide with spinel structure, where, Al usually occupies the octahedral site and Zn normally occupies the tetrahedral site of a cubic lattice (Ribeiro et al., 2010). Due to its high chemical and thermal stability and low surface acidity, ZnAl2O4 has been used as catalyst or catalytic support in several reactions such as hydrogen production (Ribeiro et al., 2010), partial oxidation of methane to syngas (López-Fonseca et al., 2012), acetylene hydrogenation (Peña et al., 1994), combustion of methane (Yazdanpanah et al., 2014) and 1,2,4-trichlorobenzene hydrodechlorination (Cesteros et al., 2000). However, a few works using ZnAl2O4 as photocatalyst for the degrading the organic pollutant molecules from aqueous solution have been reported. ZnAl2O4 particles prepared by different routes such as coprecipitation, hydrothermal and microwave-hydrothermal have been used for the textile dye degradation under solar irradiation (Foletto et al., 2012a). Recently degradation of phenol and tannery dye has been evaluated with the use of ZnAl2O4 (Anchieta et al., 2014; Battiston et al., 2014).
In this work, Procion red H-E7B dye was degraded under UV irradiation in the presence of ZnAl2O4 particles, which were synthesized by a route using chitosan as template. The effects of initial dye concentration (130, 200 and 300 mg L-1) and photocatalyst concentration (0.25, 0.50, 0.75 and 1.00 g L-1) on the dye degradation efficiency were verified. Kinetic parameters were obtained and the reaction order was determined.
II. METHODS
Procion red H-E7B, a dye extensively used in textile industry, was used as model compound. The chemical structure of the dye is showed in the work of Foletto et al. (2012a). ZnAl2O4 photocatalyst was synthesized using chitosan as template and characterized in previous work (Stringhini et al., 2014). The average crystallite size determined from X-ray diffraction was 3.6 nm. Surface area, total pore volume and average pore size were 184 m2 g-1, 0.283 cm3 g-1 and 5.66 nm, respectively (Stringhini et al., 2014). Images of ZnAl2O4 particles were obtained by digital camera and scanning electron microscope (SEM, Shimadzu SSX-550). The reaction system and experimental procedure was similar to the described by Collazzo et al. (2012b). The reactor was batch-type, consisting of a glass tube (internal diameter of 5.5 cm and 16.0 cm in height) with a medium-pressure mercury vapor lamp (80 W) fixed at the center and protected by a quartz bulb. The measured UV photon flux was 7.1×10-6 Einstein s-1 (actinometric method). The reactor was equipped with an external aluminum cylinder and a cooling system to keep the temperature constant. For the photocatalytic tests, the photocatalyst (0.25, 0.50, 0.75 or 1.00 g L-1) was added to 100 mL of dye aqueous solution at different concentrations (130, 200 or 300 mg L-1) and natural pH 6.5. Herein, the pH of aqueous solution was not changed by economic purposes related to the adsorption process. The range of dye concentration used in this work as well as the catalyst/solution ratio were based in previous studies (Sauer et al., 2005; Collazzo et al., 2012a; Collazzo et al., 2012c; Foletto et al., 2012b). Before irradiation, the suspension was stirred until reaches the adsorption equilibrium. After this step, the suspension was irradiated by the mercury vapor lamp, and aliquots were collected in predetermined time intervals. These samples were filtered before determination of dye concentration. The dye degradation efficiency was defined as % Degradation = (A0 - A)/A x 100, where A0 is the initial absorbance and A is the absorbance after the reaction, at λmax = 543 nm. The photocatalytic activity of ZnAl2O4 sample was compared with TiO2 (Degussa-P25) catalyst.
III. RESULTS AND DISCUSSION
Figure 1a shows the ZnAl2O4 dried spheres before calcination at 500 oC (obtained by digital camera). The external surface of ZnAl2O4 calcined spheres (obtained by SEM) is showed in Fig. 1b. The spheres presented particle size around 3 mm (Fig. 1a). Cracks in Fig 1b were caused by the decomposition of organic matter (chitosan), which generates particles with porous structure and high surface area (Stringhini et al., 2014).
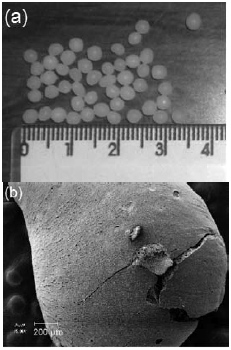
Fig. 1. Images of (a) spheres dried at room temperature before calcination at 500 oC, and (b) external surface of a sphere calcined at 500 oC.
Figure 2 shows the effect of initial dye concentration (130, 200 and 300 mg L-1) on the dye degradation efficiency. Before UV irradiation, the photocatalyst and dye solutions were stirred in the dark until reach the adsorption equilibrium. It was found in Fig. 2 that the dye degradation efficiency was favored at lower initial dye concentration. At initial dye concentration of 130 mg L-1, a complete degradation occurred at 120 min, whereas for concentrations of 200 and 300 mg L-1, it occurred at 210 and 360 min, respectively. This may occurs due to the fact that at high dye concentrations, a significant amount of light is absorbed by the dye molecules and not by the catalyst, reducing the catalytic efficiency. Furthermore, at lower concentrations, there are more water molecules which are adsorbed in ZnAl2O4, producing hydroxyl radicals and leading to a fast oxidation process. Otherwise, at higher concentrations, there are few water molecules adsorbed in the catalyst surface, consequently, the competitive adsorption effect is higher leading to a decrease in the photodegradation rate (Ishiki et al., 2005).
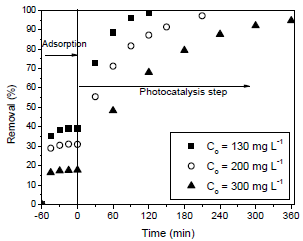
Fig. 2. Effect of initial dye concentration on the dye degradation efficiency (1 gL-1 of photocatalyst, pH=6.5 and T=25 oC).
To investigate the photocatalyst concentration effect on the dye degradation efficiency, the concentrations of 0.25, 0.50, 0.75 and 1.00 g L-1 were tested and the initial dye concentration was 130 mg L-1. The results are shown in Fig. 3.
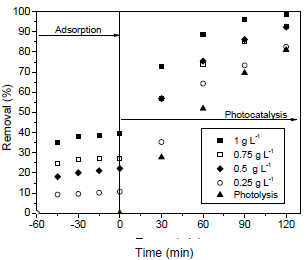
Fig. 3. Effect of photocatalyst concentration on the dye degradation efficiency (initial dye concentration of 130 mg L-1; pH = 6.5 and T = 25 oC).
As can be seen in Fig. 3, 100% of dye degradation efficiency was reached at 120 min of reaction, for the photocatalyst concentration of 1.00 g L-1. For the other photocatalyst concentrations and also for the photolysis process (without catalyst), the dye degradation was lower than 90% at 120 min of reaction. Amount of catalyst above 1.0 g L-1 was not tested herein because can increase the cost of photocatalytic process. In addition, amount of catalyst of 1.0 g L-1 has been widely used in photocatalytic processes for the degradating the organic pollutants from aqueous solution (Sauer et al., 2005; Sauer et al., 2006; Collazzo et al., 2012c; Foletto et al., 2012b). Based on the results of Figs. 2 and 3, the most promising conditions to maximize the dye degradation efficiency were initial dye concentration of 130 mg L-1 and photocatalyst concentration of 1.00 g L-1.
It was found in the literature (Yu et al., 2002; Wang et al., 2011), that many photodegradation reactions follow a pseudo-first order kinetic behavior. So, in this work, it was considered that the Procion red degradation rate (r) is proportional to its concentration in the solution, as follows:
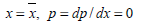 | (1) |
The solution of Eq. 1, in its linear form is given by:
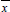 | (2) |
where, Co is the initial dye concentration (mg L-1), C is the dye concentration at any time (mg L-1) and k is the reaction rate constant (min-1).
The reaction rate constant values were determined by fitting the Equation 2 with the experimental data through linear regression. The Quasi-Newton estimation method was used and the calculations were performed using the Statistic 7.0 software (Statsoft, USA). The determination coefficient (R2) and average relative error (ARE) were used to evaluate the fit quality. The high values of R2 (R2>0.98) and the low values of ARE (ARE<10%) revealed that the Procion red degradation rate followed a pseudo-first order kinetic behavior. This can be visualized for all initial dye concentrations (Fig. 4) and photocatalyst concentrations (Fig. 5).
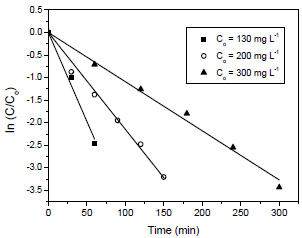
Fig. 4. Procion red degradation rate at different initial dye concentrations (1 gL-1 of photocatalyst, pH=6.5 and T=25 oC).
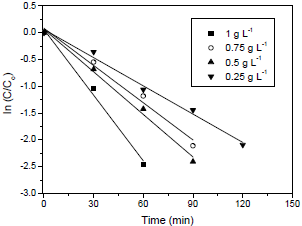
Fig. 5. Procion red degradation rate at different photocatalyst concentrations (130 mg L-1 of photocatalyst, pH = 6.5 and T = 25 oC).
The rate constant values (k) obtained by fitting the Equation 2 were 13x10-3, 18x10-3 and 40x10-3 min-1 for the initial dye concentrations of 300, 200 e 130 mg L-1. In relation to the photocatalyst concentration, the rate constant values (k) were 40x10-3, 26x10-3, 23x10-3 and 17x10-3 min-1 for the concentrations of 1.00, 0.75. 0.50 and 0.25 g L-1, respectively. These values indicated that the dye degradation rate was faster at 1.00 g L-1 of photocatalyst.
ZnAl2O4 was compared with TiO2 P-25 Degussa to degrade Procion red under de following conditions: initial dye concentration of 130 mg L-1, catalyst concentration of 1 g L-1, pH of 6.5 and temperature of 25 °C. The results are shown in Fig. 6.
Fig. 6. Comparison of ZnAl2O4 and TiO2 (P-25/Degussa) for Procion red degradation.
It can be seen in Fig. 6 that Procion red was more adsorbed on ZnAl2O4 compared with TiO2 (P-25) but, the dye was faster degraded by the use of TiO2 (P-25). The first fact could be because ZnAl2O4 have surface area of 184 m2 g-1, while TiO2 have 50 m2 g-1. The second fact can be attributed to the lower band gap of TiO2 (3.2 eV) (Foletto et al., 2012a) in relation to the band gap of ZnAl2O4 (3.8 eV) (Wang et al., 2011).
Figure 7 shows the Procion red degradation rate for ZnAl2O4 and TiO2 (P-25). The rate constant values (k) were 40x10-3 min-1 for ZnAl2O4 and 130x10-3 min-1 for TiO2. Guettai and Amar (2005) obtained k values from 3x10-3 to 143x10-3 min-1 in the photocatalytic oxidation of methyl orange in the presence of TiO2 (P-25 Degussa). Although the Procion red degradation rate by TiO2be higher than ZnAl2O4, the latter presented a considerable photocatalytic activity to degrade Procion red dye.
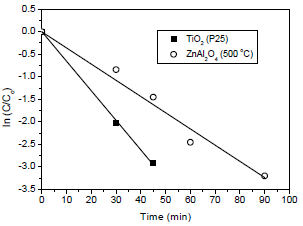
Fig. 7. Degradation rate of Procion red by ZnAl2O4 and TiO2 P-25.
III. CONCLUSIONS
ZnAl2O4 synthesized with chitosan as template was used as photocatalyst for Procion red degradation in aqueous solution. It was observed that the initial dye concentration and photocatalyst concentration influenced on the dye degradation efficiency. The more adequate conditions for the dye degradation were initial dye concentration of 130 mg L-1 and photocatalyst concentration of 1 g L-1. Under these conditions, all dye was degraded in 120 min. The dye degradation followed a pseudo-first order kinetic. ZnAl2O4 particles showed satisfactory catalytic activity for dye degradation, which can be ascribed to the high surface area. Therefore, the results indicated that ZnAl2O4 particles could be employed for the dye degradation under UV irradiation.
REFERENCES
1. Alfano, O.M. and A.E. Cassano, "Scaling-up of photoreactors: applications to advanced oxidation processes," Adv. Chem. Eng., 36, 229-287 (2009).
2. Anchieta, C.G., D. Sallet, E.L. Foletto, S.S. Silva, O. Chiavone-Filho and C.A.O. Nascimento, "Synthesis of ternary zinc spinel oxides and their application in the photodegradation of organic pollutant," Ceramics Int., 40, 4173-4178 (2014).
3. Battiston, S., C. Rigo, E.C. Severo, M.A. Mazutti, R.C. Kuhn, A. Gündel and E.L. Foletto, "Synthesis of zinc aluminate (ZnAl2O4) spinel and its application as photocatalyst," Mater. Res., 17, 734-738 (2014).
4. Cataño, F.A., S.H. Valencia, E.A. Hincapié, G.M. Restrepo and J.M. Marín, "Comparative study between tio2 and zno photocatalysis: photocatalytic degradation of cibacron yellow FN-2R Dye," Latin Am. Appl. Res., 42, 33-38 (2012).
5. Cesteros, Y., P. Salagre, F. Medina and J.E. Sueiras, "Catalytic behaviour of several Ni/NiAl2O4 catalysts in the hydrogenation of 1,2,4-trichloro-benzene and benzene to cyclohexane," Studies Surf. Sci. Catal., 130, 2069-2074 (2000).
6. Collazzo, .C., S.L. Jahn and E.L. Foletto, "Removal of direct black 38 dye by adsorption and photocatalytic degradation on TiO2 prepared at low temperature," Latin Am. Appl. Res., 42, 55-60 (2012a).
7. Collazzo, G.C., D.S. Paz, S.L. Jahn, N.L.V. Carreño and E.L. Foletto, "Evaluation of niobium oxide doped with metals in photocatalytic degradation of leather dye," Latin Am. Appl. Res., 42, 51-54 (2012b).
8. Collazzo, G.C., E.L. Foletto, S.L. Jahn and M.A. Villetti, "Degradation of Direct Black 38 dye under visible light and sunlight irradiation by N-doped anatase TIO2 as photocatalyst," J. Environm. Manage., 98, 107-111 (2012c).
9. Foletto, E.L., S. Battiston, J.M. Simões, M.M. Bassaco, L.S.F. Pereira, É.M.M. Flores and E.I. Müller, "Synthesis of ZnAl2O4 nanoparticles by different routes and the effect of its pore size on the photocatalytic process," Microp. Mesop.Mat., 163, 29-33 (2012a).
10. Foletto, E.L., S. Battiston, G.C. Collazzo, M.M. Bassaco and M.A. Mazutti, "Degradation of leather dye using CeO2-SnO2 nanocomposite as photocatalyst under sunlight," Water, Air Soil Poll., 223, 5773-5779 (2012b).
11. Foletto, E.L., J.M. Simões, M.A. Mazutti, S.L. Jahn, E.I. Muller, L.S.F. Pereira and E.M.M. Flores, "Application of Zn2SnO4 photocatalyst prepared by microwave-assisted hydrothermal route in the degradation of organic pollutant under sunlight," Ceramics Int., 39, 4569-4574 (2013).
12. Guettai, N. and H.A. Amar, "Photocatalytic oxidation of methyl orange in presence of titanium dioxide in aqueous suspension. Part II: kinetics study," Desalination, 185, 439-448 (2005).
13. Hernández-Gordillo, A., A.G. Romero, F. Tzompantzi and R. Gómez, "New nanostructured CdS fibers for the photocatalytic reduction of 4-nitrophenol," Powder Tech., 250, 97-102 (2013).
14. Ishiki, R.R., H.M. Ishiki and K. Takashima, "Photocatalytic degradation of imazethapyr herbicide at TiO2/H2O interface," Chemosphere, 58, 1464-1469 (2005).
15. Krishnan, J. and T. Swaminathan, "Kinetic modeling of a photocatalytic reactor designed for removal of gas-phase benzene: a study on limiting resistances using design of experiments," Latin Am. Appl. Res., 40, 359-364 (2010).
16. López-Fonseca, R., C. Jiménez-González, B. Rivas and J.I. Gutiérrez-Ortiz, "Partial oxidation of methane to syngas on bulk NiAl2O4 catalyst. Comparison with alumina supported nickel, platinum and rhodium catalysts," Appl. Catal. A: Gen., 437-438, 53-62 (2012).
17. Morales, G.V., E.L. Sham, R. Cornejo and E.M.F. Torres, "Kinetic studies of the photocatalytic degradation of tartrazine," Latin Am. Appl. Res., 42, 45-49 (2012).
18. Peña, J.A., J.C. Rodríguez, J. Herguido, J. Santamaría and A. Monzón, "Influence of the catalyst pretreatment on the relative rates of the main and coking reactions during acetylene hydrogenation on a NiO/NiAl2O4 catalyst," Studies Surf. Sci. Catal., 88, 555-560 (1994).
19. Ribeiro, N.F.P., R.C.R. Neto, S.F. Moya, M.M.V.M. Souza and M. Schmal, "Synthesis of NiAl2O4 with high surface area as precursor of Ni nanoparticles for hydrogen production,"Int. J. Hydrogen Energy, 35, 11725-11732 (2010).
20. Sauer, T.P., L. Casaril, E. Humeres and R.F.P.M. Moreira, "Mass transfer and photocatalytic degradation of leather dye using TiO2/UV," J. Appl. Electrochem., 35, 821-829 (2005).
21. Sauer, T.P., A.L.B. Oberziner, L. Casaril, H.J. José and R.F.P.M. Moreira, "Advanced oxidation processes applied to tannery wastewater containing Direct Black 38 - Elimination and degradation kinetics," J. Hazard. Mater., 135, 274-279 (2006).
22. Silva F.V., M.A. Lansarin and C.C. Moro, "A comparison of slurry and immobilized TiO2 in the photocatalytic degradation of phenol," Latin Am. Appl. Res., 42, 275-280 (2012).
23. Silva, L.J.V., E.L. Foletto, L.S. Dorneles, D.S. Paz, T.S. Frantz and A. Gundel, "ZnO electrodeposition onto gold from recordable compact discs and its use as photocatalyst under solar irradiation," Braz. J. Chem. Eng., 30, 155-158 (2013).
24. Stringhini, F.M., E.L. Foletto, D. Sallet, D.A. Bertuol, O. Chiavone-Filho and C.A.O. Nascimento, "Synthesis of porous zinc aluminate spinel (ZnAl2O4) by metal-chitosan complexation method," J. Alloys Compd., 588, 305-309 (2014).
25. Wang, Y., C. Feng, M. Zhang, J. Yang and Z. Zhang, "Visible light active N-doped TiO2 prepared from different precursors: Origin of the visible light absorption and photoactivity," Appl. Catal. B: Environ., 104, 268-274 (2011).
26. Yazdanpanah, M.M., A. Forret, T. Gauthier and A. Delebarre, "Modeling of CH4 combustion with NiO/NiAl2O4 in a 10 kWth CLC pilot plant," Appl. Energy, 113, 1933-1944 (2014).
27. Yu, J.C., J. Yu, W. Ho, Z. Jiang and L. Zhang, "Effects of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders," Chemistry Mat.,14, 3808-3816 (2002).
Received: March 25, 2014. Accepted: August 19, 2014.
Recommended by Subject Editor: Orlando Alfano












