Servicios Personalizados
Articulo
Latin American applied research
versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.45 no.2 Bahía Blanca abr. 2015
Ascorbic acid degradation kinetic for microwave dried basil, purslane and celery leaves
E. Demirhan, Z. Akpinar, D.K. Apar and B.Özbek
Yıldız Technical University, Department of Chemical Engineering, Davutpaşa Campus, 34210, Esenler/Istanbul, Turkey. bozbek@yildiz.edu.tr
Abstract— In this study, the goal was to evaluate the ascorbic acid degradation in basil, purslane and celery leaves during microwave drying. The effects of microwave output power and sample amount on ascorbic acid loss in basil, purslane and celery leaves were investigated. The method for the determination of ascorbic acid content was based on the reaction between ascorbic acid and 2,6-dichloroindophenol and was successfully applied to basil, purslane and celery leaves. Increasing microwave output power and decreasing sample amount led to higher degradation rates in basil, purslane and celery leaves. Ascorbic acid degradation kinetics in basil, purslane and celery leaves during microwave drying followed a first-order reaction. The activation energy values were also calculated using an exponential expression based on Arrhenius equation for degradation of ascorbic acid.
Keywords— Ascorbic Acid; Kinetic; Basil, purslane, celery leaves, microwave drying
I. INTRODUCTION
Ascorbic acid, also known as vitamin C, is a water soluble vitamin. Ascorbic acid is an essential substance found mainly in fruits and vegetables. This nutrient has vital importance in processes of oxidation and reduction in human organism participating of several metabolic reactions such as growth and repair of tissues in all parts of the body (Arrioni and De Tullio, 2002; Güçlü et al., 2005; Al Zubaidy and Khalil, 2007; Vasco et al., 2009). Due to the great importance of ascorbic acid in human beings, the quantitative analysis of ascorbic acid has gained increased significance in several areas of analytical chemistry such as pharmaceutical and food applications. It is usually selected as an index of the nutrient quality because of its labile nature compared to other nutrients in foods (Hossu and Magearu, 2007; Goula and Adamopoulos, 2006).
Ascorbic acid can be easily degraded depending on many variables such as pH, temperature, light, and presence of enzymes. Ascorbic acid degradation reactions are often responsible for important quality changes that occur during the storage of foods, limiting their shelf-life, with formation of unstable intermediate compounds, such as furfural (Al Zubaidy and Khalil, 2007; Rojas and Gerschenson, 1997). Such degradation also affects the sensory characteristics of processed food, such as flavour and colour (Rassis and Saguy, 1995; Santos and Silva, 2008).
Depending on the type of process, drying conditions, product physical properties, and time-temperature regimes used, thermal energy can cause varying degrees of loss of ascorbic acid during drying of vegetables. In general, rapid drying retains a greater amount of ascorbic acid than slow drying (Goula and Adamopoulos, 2006; Kaya et al., 2010). Several analytical methods have been reported for the determination of vitamin C using titrimetry (De Assis et al., 2009; Wunderlich et al., 2008; Kabasakalis et al., 2000), spectrometry (Arya et al., 1998), amperometry (Arya et al., 2000), voltammetry (Vazquez et al., 2012) and high resolution liquid chromatography technique (Arrioni and De Tullio, 2002; Xu et al., 2008).
Microwave drying is an alternative method compared with traditional drying processes. Microwave heating is dielectric heating but refers to the heating that takes place in a non-conductor due to polarization effects. While microwave is processing, microwaves radiate from a source in all directions. These waves carry energy, and during the drying process, material absorbs this energy and converts it to heat by polar molecules. Water is the common polar molecule and a component of foods. So, during this process, water molecules convert microwave energy to heat. Then the water molecules start to evaporate as a result of this heat, and so the material starts to dry. Microwave drying reduces the drying time and prevents food from decomposing. The previous studies performed by Demirhan and Ozbek (2010a) (for basil), Demirhan and Ozbek (2010b) (for purslane) and Demirhan and Ozbek (2011) (for celery leaves) showed that microwaves enhance the drying process of basil, purslane and celery leaves significantly in comparison to convective conditions such as hot air drying; and this technique was successfully used to dry basil, purslane and celery leaves.
The retention of ascorbic acid in dried products is assumed as a general indicator of the preservation of other less labile nutrients. Therefore, in the present study, ascorbic acid degradation of basil, purslane and celery leaves during microwave drying was investigated by using a titrimetric method. Activation energy values of degradation of ascorbic acid of basil, purslane and celery leaves were calculated by using the exponential expression based on Arrhenius equation.
II. METHODS
A. Materials
Fresh basil (Ocimum basilicum L.), purslane (Portulaca oleracea L.) and celery leaves (Apium graveolens L.) samples were purchased from a local supplier in Istanbul. They were washed and stored at 4±0.5°C at refrigerator. Before the drying experiments, the samples were taken out of the refrigerator and weighed to dry by microwave.
B. Drying equipment and drying method
Microwave drying experiments for basil, purslane and celery leaves were performed in a domestic digital microwave oven (Arcelik MD 594, Turkey). During drying experiments, each sample was put on the rotating glass plate and placed at the center of the oven. Moisture loss was periodically measured. Three replications of each experiment were performed according to a preset microwave power level and time schedule, and the data given are an average of these results. The reproducibility of the experiments was within the range of ±5%. The microwave power was applied until the weight of the sample reduced to a level corresponding to moisture content of about 0.1 g water/g dry base. All weighing processes were completed in less than 10 s during the drying process (Demirhan and Ozbek, 2010a; Demirhan and Ozbek, 2010b; Demirhan and Ozbek, 2011).
In the present study, effects of microwave output power and sample amount on ascorbic acid degradation of basil, purslane and celery leaves were investigated at different microwave output powers (180, 360, 540, 720 and 900 W) and different sample amounts (25, 50, 75 and 100 g).
C. Determination of Ascorbic Acid
Ascorbic acid (AA) was determined by titrimetric method defined by AOAC (1980). Dried samples were blended at high speed in 100 ml of 3% (w/v) metaphosphoric acid/acetic acid solution and then filtered. Preparations of the sample solutions were performed just before the titration. Then 5 ml sample solution was rediluted with 30 ml distilled water. This solution was titrated with 2,6-dichloroindophenol solution until the solution turned pink and then kept for at least 15 sec, and the volume of titrant was recorded. The volumes of titrant used for the standard L-ascorbic acid solution and sample solution were calculated by subtracting the volume of the titrant used for the blank solution from those of the standard and sample solutions, respectively. These measurements were repeated three times and averages were taken. The reproducibility of the measurements made for ascorbic acid content was within the range of ±5%. Then, the ascorbic acid content of fresh samples of basil, purslane and celery leaves were found as 21.6, 21.2 and 30 mg L-ascorbic acid/100 g, respectively.
D. Kinetic model for Ascorbic acid degradation
In order to determine ascorbic acid degradation during drying process as a function of drying time, zero-order kinetic model (Eq. 1) and first-order kinetic model (Eq. 2) were used (Erenturk et al., 2005; Gamboa-Santos et al., 2014);
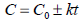 | (1) |
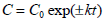 | (2) |
where; C0 is the initial value of ascorbic acid (mg AA/100 g), C is the ascorbic acid value at pre-specified time (mg AA/100 g), k is the degradation rate constant (min-1) and t is the drying time (min).
E. Computational work
The regression analysis was performed using the MATHLAB 5.0 computer program. The goodness of fit of the tested mathematical model to the experimental data was evaluated from the coefficient of determination (R2) and the standard error (σ) between the predicted and experimental values. The standard error (σ) can be calculated as follows;
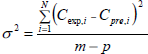 | (3) |
where Cexp,i is the experimental data, Cpre,i is the predicted data, m is the number of observations and p is number of constants.
III. RESULTS AND DISCUSSIONS
The effect of microwave output power and sample amount on ascorbic acid content
Nutritional quality deterioration during drying of basil, purslane and celery leaves was assessed in terms of ascorbic acid content, which was selected due to its temperature sensitivity. The effect of microwave output power on ascorbic acid content of basil, purslane and celery leaves was investigated at five different power levels; 180, 360, 540, 720 and 900 W; using samples of 25 g
The graphical representations of the predicted and experimental ascorbic acid content of basil, purslane and celery leaves were shown in Fig. 1, for various microwave output powers applied. As seen from this figure, as the microwave output power increased from 180 to 900 W, the content of ascorbic acid for basil, purslane and celery leaves decreased. This can be explained by higher energy transferred to per unit of sample mass which ranged from 7.2 W/g to 36 W/g; which resulted in higher product temperature of samples, and as a result, a greater degradation rate of ascorbic acid was observed for samples at 900 W microwave output power. At this microwave output power, the ascorbic acid loss was found as 95%, 84% and 95% for basil, purslane and celery leaves, respectively.
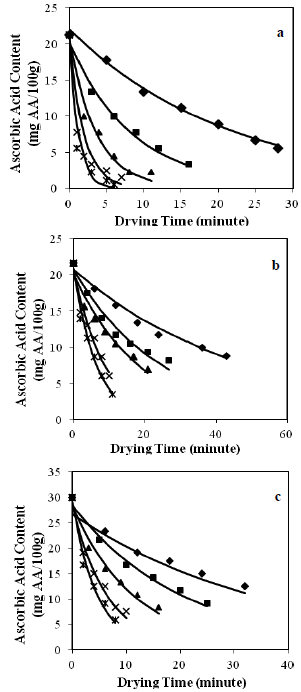
Figure 1. Degradation of ascorbic acid at various microwave output powers a) basil leaves b) purslane leaves c) purslane leaves (sample amount 25 g) ( 180 W,
180 W,  360 W
360 W  540 W, × 720 W,
540 W, × 720 W,  900 W, - kinetic model).
900 W, - kinetic model).
The increase of ascorbic acid degradation with high product temperature was reported for drying of several foods like spinach (Dadali and Özbek, 2009), pepper (Vega-G´lvez et al., 2009), papaya (Kurozawa et al., 2014), Hayward kiwifruits (Kaya et al., 2010), pineapple (Ramallo and Mascheroni, 2012), strawberries (Gamboa-Santos et al., 2014), pear (Mrad et al., 2012) and tomato (Marfil et al., 2008). Dadali and Özbek (2009) were observed that the ascorbic acid content decrease with increase in microwave output power. Mrad et al. (2012) evaluated the ascorbic acid degradation during drying of pear at different air temperatures and; the ascorbic acid loss was found as 70% for pears dried at 70°C. Similarly, Vega-Galvez et al (2009) obtained highest value of ascorbic acid loss (98.2%) at high temperature value of 90°C. Marfil et al. (2008) also observed that higher drying temperatures have increased vitamin C degradation rate on peeled dried tomatoes.
In literature, no study was found about investigation of the effect of sample amount on ascorbic acid content of basil, purslane and celery leaves undergoing microwave treatment. Therefore, in this work, the ascorbic acid degradation of basil, purslane and celery leaves at various sample amounts, ranging from 25 to 100 g were studied at constant microwave output power of 360 W. The predicted and experimental data of ascorbic acid content of basil, purslane and celery leaves were shown in Figure 2. As seen from figure, as the sample amount increased from 25 to 100 g, the degradation of ascorbic acid of basil, purslane and celery leaves samples decreased. The same behavior was obtained by Dadali and Özbek (2009) for spinach leaves undergoing microwave treatment.
Figure 2. Degradation of ascorbic acid at various sample amounts a) basil leaves b) purslane leaves c) celery leaves (microwave output power 360 W) ( 25 g,
25 g,  50 g, × 75 g,
50 g, × 75 g,  100 g, - kinetic model).
100 g, - kinetic model).
Ascorbic acid degradation kinetics
For mathematical modeling of degradation of ascorbic acid of basil, purslane and celery leaves dried at different microwave output powers and sample amounts, empirical zero-order kinetic (Eq. 1) and first-order kinetic model (Eq. 2) were used. Between these two models, first-order kinetic model was found the most suitable one for all the experimental data with higher value for the coefficient of determination and lower standard error compared with zero-order kinetic model. The estimated kinetic parameters of these models and the statistical values of coefficients of determination R2 and the standard error (σ) were represented in Table 1 and 2 for basil, purslane and celery leaves.
Table 1. The estimated kinetic parameters and the statistical values of kinetic models for basil, purslane and celery leaves at various microwave output powers
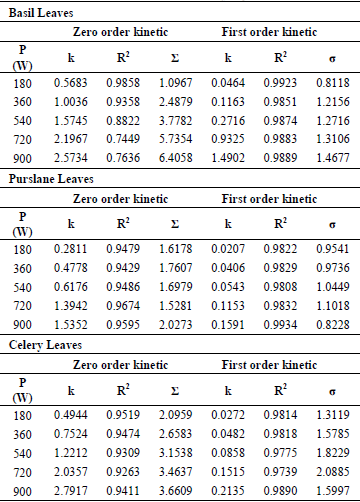
Table 2. The estimated kinetic parameters and the statistical values of kinetics model for basil, purslane and celery leaves at various sample amounts
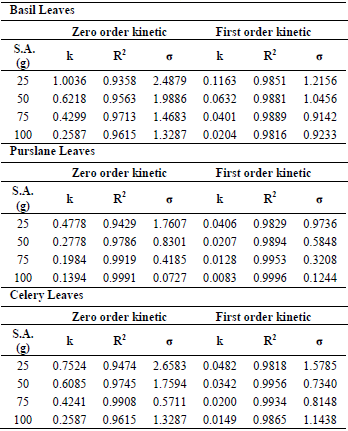
As the microwave output increased, the kinetic rate constants for basil, purslane and celery leaves were increased from 0.0464 to 1.4902 min-1, from 0.0207 to 0.1591 min-1, from 0.0272 to 0.2135 min-1, respectively. On the other hand, the kinetic rate constants for basil, purslane and celery leaves were decreased as the sample amount increased. The results obtained were in agreement with studies published in the literature and, the first order kinetic for ascorbic acid degradation were reported for different fruits by Gamboa-Santos et al. (2014), Demiray et al. (2013), Marfil et al. (2008), Kurozawa et al. (2014).
Estimation of activation energy values
In this study, as the temperature is not measurably variable in the standard microwave oven used for drying process, the Arrhenius equation was used in a modified form to illustrate the relationship between the kinetic rate constant and the ratio of the microwave output power to sample amount instead of the temperature for calculation of the activation energy. After evaluation of the data, the dependence of the kinetic rate constant on the ratio of microwave output power to sample amount was represented with an exponential equation (Eq. 4) derived by Dadali et al. (2007);
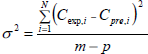 | (4) |
where k is the degradation rate constant obtained by first-order kinetic model (min-1), k0 is the pre-exponential constant (min-1), Ea is the activation energy (minimum energy required for ascorbic acid degradation during microwave drying; W.g-1), P is microwave output power (W) and m is the mass of raw sample (g).
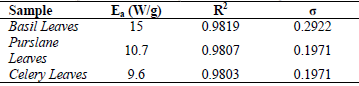
The activation energy values for basil, purslane and celery leaves, corresponding values of coefficients of determination R2 and the standard error (σ) of the model proposed were given in Table 3. As can be seen from the table, the activation energy values for basil, purslane and celery leaves were found as 15 W/g, 10.7 W/g and 9.6 W/g, respectively.
Table 3. The activation energy values calculated for ascorbic acid degradation of basil, purslane and celery leaves
IV. CONCLUSIONS
The kinetic degradation of ascorbic acid for basil, purslane and celery leaves was evaluated during microwave drying. The effects of various microwave output powers and various sample amounts were investigated. It was observed that as the microwave output power increased the content of ascorbic acid of basil, purslane and celery leaves decreased due to high temperature occurred in the dried product depending on higher levels of microwave energy applied. Moreover, it was found that as the sample amount increased, ascorbic acid degradation of basil, purslane and celery leaves was decreased as well.
After evaluation of the experimental data, an empirical first-order kinetic model was used for the degradation of ascorbic acid for basil, purslane and celery leaves undergoing microwave heat treatment; and moreover the activation energy values were found as 15 W/g, 10.7 W/g and 9.6 W/g for basil, purslane and celery leaves, respectively.
REFERENCES
1. Al-Zubaidy, M.M.I. and R.A. Khalil, "Kinetic and prediction studies of ascorbic acid degradation in normal and concentrate local lemon juice during storage," Food Chem., 101, 254-259 (2007).
2. AOAC, Official methods of Analysis of the Association of Official Analytical Chemists, 13th edition, Association of Official Analytical Chemists, Washington, DC (1980).
3. Arrigoni, O. and M.C. De Tullio, "Ascorbic acid: much more than just an antioxidant," Biochim. et Biophy. Acta, 1569, 1-9 (2002).
4. Arya, S.P., M. Mahajan and P. Jain, "Non-spectrophotometric methods for the determination of vitamin C," Anal. Chim. Acta, 417, 1-14 (2000).
5. Arya, S.P., M. Mahajan and P. Jain, "Photometric methods for the determination of vitamin C," Anal. Sci., 14, 889-895 (1998).
6. Dadali, G., D.K. Apar and B. Ozbek, "Microwave drying kinetics of okra," Dry. Technol., 25, 917-924 (2007).
7. Dadali, G. and B. Ozbek, "Kinetic thermal degradation of vitamin C during microwave drying of okra and spinach," Int. J. Food Sci. Nutr., 60, 21-31 (2009).
8. De Assis, S.A., J.C.R. Vellosa, I.L. Brunetti, N.M. Khalil, K.M.D.C. Leite, A.B.G. Martins and O.M.M.D. Oliveria, "Antioxidant activity, ascorbic acid and total phenol of exotic fruits occurring in Brazil," Int. J. Food Sci. Nutr., 60, 439-448 (2009).
9. Demirhan, E. and B. Ozbek, "Microwave drying characteristics of basil," J. Food Process. Preserv., 34, 476-494 (2010a).
10. Demirhan, E. and B. Ozbek, "Drying Kinetics and Effective Moisture Diffusivity of Purslane Undergoing Microwave Heat Treatment," Korean J. Chem. Eng., 27, 1377-1383 (2010b).
11. Demirhan, E. and B. Ozbek, "Thin-Layer Drying Characteristics and Modelling of Celery Leaves Undergoing Microwave Treatment," Chem. Eng. Comm., 198, 957-975 (2011).
12. Demiray, E., Y. Tulek and Yilmaz, "Degradation kinetics of lycopene, b-carotene and ascorbic acid in tomatoes during hot air drying," LWT - Food Sci. Technol., 50, 172-176 (2013).
13. Erentürk, S., M.S. Gulaboglu and S. Gültekin, "The effects of cutting and drying medium on the Vitamin C content of rosehip during drying," J. Food Eng., 68, 513-518 (2005).
14. Gamboa-Santos, J., R. Megías-Pérez, A.C. Soria, A. Olano, A. Montilla and M. Villamiel, "Impact of processing conditions on the kinetic of vitamin C degradation and 2-furoylmethyl amino acid formation in dried strawberries," Food Chem., 153, 164-170 (2014).
15. Goula, A.M. and K.G. Adamopoulos, "Retention of Ascorbic Acid during drying of tomato halves and tomato pulp," Dry. Technol., 24, 57-64 (2006).
16. Güçlü, K., K. Sözgen, E. Tütem, M. Özyürek and R. Apak, "Spectrophotometric determination of ascorbic acid using copper(II)-neocuproine reagent in beverages and pharmaceuticals," Talanta, 65, 1226-1232 (2005).
17. Hossu, A. and V. Magearu, "Determination of vitamin C in pharmaceutical products with physico-chemical and bioanalytical technics," Rom. Biotech. Lett., 9, 1497-1504 (2007).
18. Kabasakalis, V., D. Siopidou and E. Moshatou, "Ascorbic acid content of commercial fruit juices and its rate of loss upon storage," Food Chem., 70, 325-328 (2000).
19. Kaya, A., O. Aydin and S. Kolayli, "Effect of different drying conditions on the vitamin C (ascorbic acid) content of Hayward kiwifruits (Actinidia deliciosa Planch)," Food Bioprod. Process., 88, 165-173 (2010).
20. Kurozawa, L.E., I. Terng, M.D. Hubinger and K.J. Park, "Ascorbic acid degradation of papaya during drying: Effect of process conditions and glass transition phenomenon," J. Food Eng., 123, 157-164 (2014).
21. Marfil, P.H.M., E.M. Santos and V.R.N. Telis, "Ascorbic acid degradation kinetics in tomatoes at different drying conditions," LWT - Food Sci. Technol., 41, 1642-1647 (2008).
22. Mrad, N.D., N. Boudhrioua, N. Kechaou, F. Courtis and C. Bonazzi, "Influence of air drying temperature on kinetics, physicochemical properties, total phenolic content and ascorbic acid of pears," Food Bioprod. Process., 90, 433-441 (2012).
23. Ramallo, L.A. and R.H. Mascheroni, "Quality evaluation of pineapple fruit during drying process," Food Bioprod. Process., 90, 275-283 (2012).
24. Rassis, D. and I. Saguy, "Oxygen effect nonenzymatic browning and vitamin C in commercial citrus juices and concentrate," Lebensm. Wiss. Technol., 28, 285-290 (1995).
25. Rojas, A. and L. Gerschenson, "Ascorbic acid destruction in sweet aqueous model systems," Lebensm. Wiss. Technol., 30, 567-572 (1997).
26. Santos, P.H.S. and M.A. Silva, "Retention of Vitamin C in Drying Processes of Fruits and Vegetables-A Review," Dry. Technol., 26, 1421-1437 (2008).
27. Vasco, C., J. Avila, J. Ruales, U. Svanberg and A. Kamal-Eldin, "Physical and chemical characteristics of golden-yellow and purple-red varieties of tamarillo fruit (Solanum betaceum Cav.)," Int. J. Food Sci. Nutr., 60, 278-288 (2009).
27. Vazquez, D., M. Tascon and L. Deban, "Determination of ascorbic acid in commercial juices on a modified carbon paste electrode by using a Taguchi Experimental Design," Food Anal. Method., 5, 441-447 (2012).
28. Vega-Gálvez, A., K. Di Scala, K. Rodríguez, R. Lemus-Mondaca, M. Miranda, J. López and M. Perez-Won, "Effect of air-drying temperature on physico-chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capsicum annuum, L. var. Hungarian)," Food Chem., 117, 647-653 (2009).
29. Wunderlich, S.M., C. Feldman, S. Kane and T. Hazhin, "Nutritional quality of organic, conventional, and seasonally grown broccoli using vitamin C as a marker," Int. J. Food Sci. Nutr., 59, 34-45 (2008).
30. Xu, G., D. Liu, J. Chen, X. Ye, Y. Ma and J. Shi, "Juice components and antioxidant capacity of citrus varieties cultivated in China," Food Chem., 106, 545-551 (2008).
Received: January 9, 2014.
Accepted: October 16, 2014.
Recommended by Subject Editor: María Luján Ferreira












