Servicios Personalizados
Articulo
Latin American applied research
versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.45 no.2 Bahía Blanca abr. 2015
Effect of oil extraction with supercritical CO2 and organic solvents on antioxidant capacity and total phenolic content of Mucuna meal
V.A.S. Garcia†, C.O.T. Lemos‡, D. Mantovani*, M.L. Corazza*, E.F. Zanoelo*, C. Da Silva§, L. Cardozo-Filho‡
† Department of Agronomy, Maringá State University, Maringá, 87020-900, PR, Brazil. garcia.vitoraugusto@gmail.com
‡ Department of Chemical Engineering, Maringá State University, Maringá, 87020-900, PR, Brazil. cardozodequem@yahoo.com.br
§ Department of Technology, Maringá State University, Maringá, 87506-370, PR, Brazil. camiladasilva.eq@gmail.com
* Department of Chemical Engineering, Federal University of Paraná, Curitiba, 81531-990, PR, Brazil. everton.zanoelo@ufpr.br
Abstract— The antioxidant capacity and total phenolic content of defatted and raw meals of three different varieties of Mucuna seeds (aterrima, cinerium and deeringiana) were investigated. Oil extraction was performed with supercritical CO2, dichloromethane and hexane. Supercritical fluid extraction was carried out at 313 K and 333 K in the pressure range from 15 MPa to 25 MPa. The DPPH (2,2-diphenyl-1-picryl-hydrazyl) assay always revealed a positive influence of oil removal on the antioxidant activity of the meals (i.e.; a negative influence on EC50) for all the considered varieties of Mucuna. The highest content of total phenols in the defatted material was approximately 18-26 % higher than those found in the raw meals (≈4.3-5.5 g GAE/100 g) when examining samples of the same Mucuna variety. A non negligible role of the oil extraction method in the total phenolic content of defatted Mucuna was evidenced in 2/3 of the examined varieties. The oil extraction yields were determined at all the investigated conditions, but the best results were obtained when using the organic solvents (≈5.2-7.4 %).
Keywords— Oil Extraction; Antioxidant Capacity; Phenol; Carbon Dioxide; Organic Solvents.
I. INTRODUCTION
Oxidation of chemical compounds is responsible for several undesirable characteristics in food products (Shahidi and Wanasundara, 1992) with detrimental impact on food quality. The kinetics of oxidation essentially involves the formation, propagation and conversion of free radicals to stable oxygenated compounds (Kapoor et al., 2009). Synthetic antioxidants (e.g.: butylated hydroxyanisole, butylated hydroxytoluene, propyl gallate) have been extensively used by food manufacturers as a strategy to break down free radical chain reactions (Yildirim et al., 2001; Kapoor et al., 2009), which naturally take place during the final stage of the aforementioned mechanism of oxidation. However, it is believed that the regular intake of food containing such additives increases the risk of cellular damages in the human body, whose final consequence may be a major incidence of several human diseases (Shahidi and Wana-sundara, 1992; Yildirim et al., 2001).
To deal with this drawback, the use of natural antioxidants from different plants has been suggested (Lee et al., 2004; Zura-Bravo et al., 2013). In fact, many of these compounds are already widely used in the food industry as potential inhibitors of lipid peroxidation, with the general purpose of increasing the shelf life of processed foods (Kapoor et al., 2009; Scherer and Godoy, 2009). Among the different families of chemical species with these properties, the antioxidant nature of phenols have been particularly explored (Yildirim et al., 2001; Batiston et al., 2013). It essentially happens because they are found in almost all plant systems (Yildirim et al., 2001; Zura-Bravo et al., 2013), thus also in the Mucuna (Longhi et al., 2011; Adebowale et al., 2005; Vadivel and Biesalski, 2012).
Mucuna is a plant of the Fabaceae family typically found in tropical regions (Longhi et al., 2011; Umoren et al., 2008). It is well-known that Mucuna seeds have an important antioxidant activity (Longhi et al., 2011; Vadivel et al., 2011). However, their antioxidant properties have been almost exclusively attributed to the moderate contents of L-Dopa (Rajeshwar et al., 2005; Bonis et al., 2010) found in their seeds (Vadivel and Pugalenthi, 2008), while the role of phenols typically present in the same part of the plant (e.g.: Longhi et al., 2011; Adebowale et al., 2005) has not been investigated enough. Anyway, because of the proven low solubility of L-Dopa in supercritical CO2 (Garcia et al., 2012; Garcia et al., 2013), supercritical fluid extraction (SFE) has been suggested to remove the oil present in the seeds of Mucuna (≈8.3-10.7 %, w/w) (Adebowale et al., 2005) as a strategy to increase the content of L-Dopa in the Mucuna meal (Garcia et al., 2012; Garcia et al., 2013). The practical interest in keeping L-Dopa in the Mucuna meal (Garcia et al., 2012) combined with the well-known benefits of SFE (e.g.: Ruetsch et al., 2003; Cardozo Jr. et al., 2007) makes the use of supercritical fluid a promising strategy of oil removal to produce defatted Mucuna meal. However, the influence of applying such a method of oil extraction on antioxidant capacity and on the level of different bioactive compounds (e.g.: total phenols) in the processed meal has not been properly considered.
Based on this framework, the main aim of the current manuscript is to determine experimentally the antioxidant capacity and total phenolic content of three different varieties of Mucuna meals defatted by supercritical carbon dioxide. However, in order to check for the effect of the method of oil extraction on the investigated responses, an additional set of extraction experiments was carried out in a Soxhlet extractor at the boiling point of dichloromethane and hexane. The influence of oil removal on antioxidant capacity and total phenol content was also considered. It was essentially done by examining samples of raw seeds and defatted meals of Mucuna in terms of the investigated variables.
II. METHODS
Seed samples of Mucuna (M. aterrima, M. cinerium and M. deeringiana) were obtained from Pró Sementes (São Paulo, Brazil). All the seeds were milled using an electric mill (IKA, model A11 B, São Paulo, Brazil) prior to taking them to a mechanical shaker (Bertel, series 1.0, São Paulo, Brazil), where a set of standard screens (Tyler series) was arranged in a stack to classify the examined samples in terms of size. Only the particles with an average diameter of 0.6 mm (mesh 30) were used in the experiments. Because it is well-known that moisture content has a significant effect on oil extraction (Hofmann et al., 2012), this parameter was determined gravimetrically by placing the seed samples in an oven at 80 ± 2 °C for a drying time necessary to have constant weight. The moisture content of the three different investigated varieties of Mucuna was approximately between 9.1 and 10.2 %.
Technical-grade carbon dioxide (95 % purity) from Air Liquide (Paraná, Brazil) was used for supercritical extraction. For conventional extraction both dichloromethane (99.6 %) and n-hexane (99.6 %) were from Merck (São Paulo, Brazil). To determine the antioxidant capacity, 2,2-diphenyl-1-picryl-hydrazyl (DPPH) and butylated hydroxytoluene (BHT) were purchased from Sigma Aldrich, while methanol and ethanol were from Fmaia (São Paulo, Brazil). For total phenolic the Folin-Denis reagent was purchased from Sigma Aldrich, calcium carbonate 14 % from Synth (São Paulo, Brazil) and gallic acid was purchased from Vetec (Rio de Janeiro, Brazil). Water was purified with a Milli-Q system from Millipore (MA, USA).
The experiments were performed in a "home-made" apparatus that is described in detail in the literature (Garcia et al., 2012; Garcia et al., 2013). It basically consisted of a solvent reservoir, two thermostatic baths, two syringe pumps (Teledyne ISCO 500), a 166.5 mL jacketed extraction vessel, an absolute pressure transducer (Smar, LD301) (0.12 bar precision) equipped with a portable programmer (Smar, HT 201) and a collector vessel made of glass. Amounts of around 60 g of finely comminuted seeds of Mucuna in the moisture content range from 9.1 to 10.2 % were placed in the extraction vessel. The solvent was pumped at a constant flow rate of 3 mL/min into the bed, which was supported by two 200 mesh wire disks at both ends, and kept in contact with the plant matrix for at least 30 min to allow system stabilization. Then, the extract was collected by opening the metering valve and needle valve; the mass of the extracted oil was measured, and the glass tube was reconnected to the equipment. The procedure was repeated (10-17 times) (300-510 min) until the obtained mass of extracted oil was no more significant.
The extraction with organic solvents was basically considered to check for the influence of oil extraction method on antioxidant capacity and total phenolic content of Mucuna meals. The experiments were carried out in a Soxhlet extractor (Satelit, Brazil) fed with approximately 30 g of Mucuna seeds kept at the boiling point of dichloromethane (313.15 K) and hexane (333.15 K) for approximately 480 min (Corso et al., 2010).
The DPPH (2,2-diphenyl-1-picryl-hydrazyl) spectrophotometric assay based on the methodology of Brand-Williams et al. (1995) with modifications (Duarte-Almeida et al., 2006), was used to determine the antioxidant capacity of the Mucuna meals. The free radical scavenging efficiency of the antioxidant compounds found in the meals was essentially measured by monitoring the color of the solution that typically changes from violet to yellow when the concentration of DPPH radical is reduced (Mensor et al., 2001).
Samples of 0.1 g of defatted Mucuna meal and 5 mL of ultrapure Milli-Q water were mixed and taken to a sonication bath (Unique 1400A) for 5 min. An aliquot of 150 μL of solution samples at different concentrations was added to 2850 μL of DPPH methanol solution. To prepare the negative control, 150 μL of distilled water (instead of a solution sample) was added to the DPPH methanol solution. 150 μL of an ethanol-BHT solution 0.02 % was used as positive control. The mixtures were taken in a dark room at the ambient temperature for 1 h. Then, the absorbance was measured at 515 nm with an spectrophotometer (Shimadzu UV-1203). The experiments with an antioxidant concentration equal to 100 mg/mL were run in triplicate, and the average value of antioxidant capacity was recorded.
The concentration of antioxidant to have an AC (antioxidant capacity) equal to 50% is referred to as EC50. It was calculated at all the investigated conditions by a linear regression considering a set of 7 results of antioxidant capacity in the antioxidant concentration (EC) range from 13.33 to 100 μg/mL. This analysis was performed by using the software Excel 2007 (Microsoft Co.).
The method described by Meda et al. (2005) with modifications was employed to determine the total phenolic content. A mixture of 0.1 g of Mucuna meal and 5 mL of ultrapure Milli-Q water was taken to a sonication bath (Unique 1400A) for 5 min. An aliquot of 50 μL of this solution was added in a cuvette containing 450 μL of ultrapure Milli-Q water and 2.5 mL 10% Folin-Denis in ultrapure Milli-Q water, where 5 min later it was also introduced 2.0 mL of a fresh solution of sodium carbonate 14 %. The mixture was kept in the dark for 2 h before measuring its absorbance at 760 nm in a spectrophotometer (Shimadzu UV-1203). An identical solution was prepared, but without adding the meal to be used as a blank solution. The concentration of total phenols was calculated with a calibration curve (R2=0.999) built with standards of pure gallic acid. Because of it, total phenolic content was expressed as g of gallic acid equivalents (GAE) per 100 g of Mucuna meal. All the experiments were performed in triplicate.
Analysis of variance (ANOVA) and comparison between mean values with the Tukey's test at a 0.95 probability level (p<0.05) were made by using the SAS 9.1 software (SAS Institute).
III. RESULTS AND DISCUSSION
The extraction yields and the operating conditions to determine them are summarized in Table 1. It can be promptly observed that an increase in pressure by considering the same temperature enhances the yield of SFE for all the varieties of Mucuna. The reason for such a behavior is the increase of density and solvating power of the supercritical fluid (Brunner, 1994). Because of the significant effect of pressure, the maximum oil yields obtained with supercritical CO2 (2.90 %, 2.74 % and 4.56 %) were always from the experiments at the highest pressure (i.e.; 25 MPa). It is also important to observe that the influence of temperature on extraction yields is not negligible since the results obtained when this factor was changed are statistically different (p<0.05) for all the considered pressures and varieties of Mucuna.
Table 1. Oil extraction yields at the examined experimental conditions.
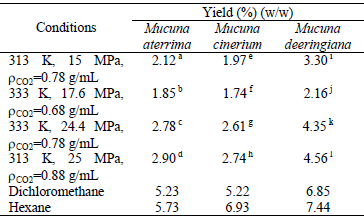
Averages followed by the same uppercase letters do not differ statistically by Tukey test at p<0.05.
An already well-explored (e.g.; Benelli et al., 2010; Garcia et al., 2012), but still important aspect emerged from Table 1, is that the yields when involving organic solvents were approximately 50 % to 150 % higher than the highest yields obtained with supercritical CO2. Such a result is essentially attributed to the solute-solvent interactions and temperature of extraction. Further information regarding the influence of variables on the concentration of oil in the extract at equilibrium is available in the literature (Garcia et al., 2012; Garcia et al., 2013).
The kinetics of oil extraction with CO2 at the same conditions considered here was already investigated (Garcia et al., 2012). The kinetic extraction curves always presented an initial period of linear increase of extraction yield, but as time passed the rate of extraction was reduced and the yield approached a fixed value close to equilibrium. The influence of solvent density on the kinetics was markedly important. Details on the kinetics of oil removal are presented in the aforementioned reference.
Chemical analyses of Mucuna oil obtained at extraction conditions identical to those applied in the current study were also previously performed (Garcia et al., 2012). However, such a chemical characterization was only in terms of fatty acids and free glycerol compounds, that is, quantification of total phenols was not performed.
At this point is important to remember that the main interest of this investigation was to examine the antioxidant activity and total phenolic content in defatted meal. So, as already described, the DPPH free radical scavenging capacity test was employed to evaluate the antioxidant capacity of the defatted Mucuna meals. Table 2 presents the maximum values of AC, which were always obtained for the highest concentration of antioxidant (i.e.; EC=100 μg/mL).
Table 2. Antioxidant capacity of raw Mucuna and defatted Mucuna meal at EC equal to 100 μg/mL.
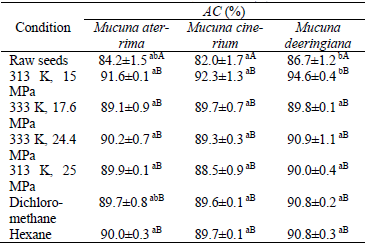
Averages followed by the same lowercase letter in the line and uppercase letters in the column do not differ statistically by Tukey test at p<0.05.
On the whole, according to these results, there are no significant differences among the antioxidant capacity of the three different Mucuna varieties. The only exceptions are: i) the antioxidant capacity of the raw meal of Mucuna deeringiana (86.7 ± 1.2 %) that is statistically higher than the same property for Mucuna cinerium (82.0 ± 1.7 %); ii) the AC of Mucuna deeringiana defatted with supercritical CO2 at 313 K and 15 MPa whose value (94.6 ± 0.4 %) is higher than those found for both of the other varieties of Mucuna early treated at the same operating conditions to oil removal.
In spite of the above discussion, the most important result emerged from Table 2 is the higher antioxidant capacity of defatted meal obtained by SFE at 313 K and 15 MPa when compared to the analogous data of the raw meals. Such a finding was noticed for all the investigated varieties of Mucuna. Because Table 2 summarizes the results of the investigated parameter for the entire set of defatted meals, one promptly realizes that the investigated methods of oil extraction, as well as the different temperature and pressure applied during SFE, have a negligible impact on the antioxidant capacity. An analysis limited to these aspects leads one to believe that there is no advantage of applying supercritical CO2 to increase the antioxidant capacity of defatted meals. However, when taking the many other benefits of applying SFE into account, such as the non-toxic nature of supercritical solvents, high diffusivities and low temperatures of extraction (Ambrogi et al., 2003; Ruetsch et al., 2003; Cardozo Jr. et al., 2007) the use of supercritical CO2 becomes much more attractive for the current purpose (i.e.; increase the AC of defatted meals) than the conventional extraction with organic solvents.
The amount of Mucuna meal required to reduce the DPPH radical to a half of its initial concentration, that is, the concentration of raw antioxidant to have an antioxidant capacity equal to 50 % (a parameter usually referred to as EC50) was also determined and presented in Fig. 1 at all the examined conditions.
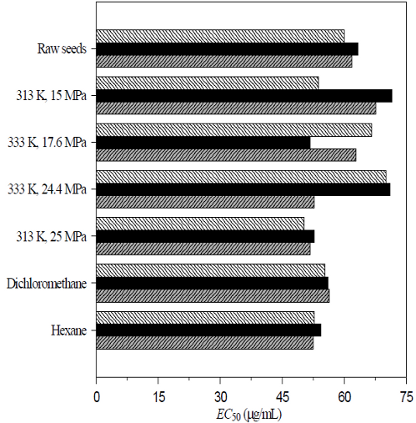
Figure 1. EC50 (concentration required to obtain 50% inhibition) of Mucuna seeds and defatted Mucuna meal varieties ( ) aterrima, (
) aterrima, ( ) cinerium and (
) cinerium and ( ) deeringiana.
) deeringiana.
The data of EC50 were tuned on a large set of experimental measurements of AC by involving Eq. (1) (i.e.; 7 different concentrations of antioxidant × 3 replications at identical conditions). So, a more accurate analysis of the influence of oil extraction method on antioxidant capacity should emerge from these results. The reliability of Eq. (1) is clearly evidenced in Fig. 2, for the case of antioxidant capacity of Mucuna deeringiana meal defatted with dichloromethane.
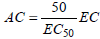 | (1) |
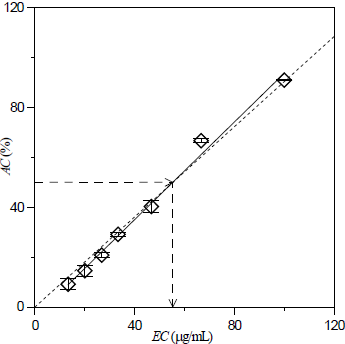
Figure 2. Antioxidant capacity as a function of concentration of Mucuna deeringiana meal preliminary taken to an operation of oil extraction with dichloromethane at 313 K. EC50=55.3 μg/mL; solid line: linear equation tuned on experimental data and used to calculate EC50; short dashed line: Equation (1); bar errors: based on three different measurements of AC at the same concentration.
It is possible to observe in Fig. 1 that the EC50 for the raw meals of Mucuna (59.9 to 63.2 μg/mL) are higher than those obtained with SFE at 313 K and 15 MPa (50.5 to 53.5 μg/mL). Such a behavior confirms the major finding revealed in Table 2, that is, the operation of oil removal with supercritical CO2 is an efficient procedure to increase the antioxidant capacity of Mucuna meal.
The results of EC50 presented in Fig. 1 also show that the rise of pressure at 313 K (which is the highest change of pressure currently observed, ΔP=10 MPa) caused an increase in the EC50 values of defatted meal obtained by SFE for Mucuna aterrima (51.5 to 67.7 μg/mL), Mucuna cinerium (53.2 to 71.6 μg/mL) and Mucuna deeringiana, (50.5 to 53.7 μg/mL). In other words, it essentially indicates that the increase of solvent density (0.78 g/mL to 0.88 g/mL) had a detrimental effect on the EC50. This finding was already reported in the literature when examining the antioxidant capacity of extracts of plants obtained by using supercritical CO2 as solvent (Gelmez et al., 2007; Lemos et al., 2012), which is a result strictly connected to the solubility of antioxidant compounds.
Results of EC50 for extracts of Mucuna are found in the literature, but many of them were obtained at operating conditions quite different than those currently considered (Rajeshwar et al., 2005; Kumar et al., 2010; Longhi et al., 2011; Du and Li, 2012). It means that a direct comparison between the values of EC50 in Figure 1 and the available data for the same parameter is not recommended. Moreover, it is necessary to consider that the antioxidant capacity of raw natural materials is not only dependent on the method of obtaining the extract.
For instance, it may depend on genetic characteristics and conditions of cultivation (Longhi et al., 2011). Anyway, these data are important to verify the general role of Mucuna as a source of natural antioxidant species.
Du and Li (2012) examined the antioxidant activity of different antioxidant compounds obtained from ethanol extracts of Mucuna sempervirens leaves and reported values of EC50 from 6.9 to 10.6 μg/mL. From a similar investigation (Kumar et al., 2010), raw extracts of whole plant of Mucuna pruriens obtained with petroleum ether, ethyl acetate and methanol as solvents presented EC50 close to 1230 μg/mL, 420 μg/mL, 1030 μg/mL, respectively. Longhi et al. (2011) had estimated that the EC50 for the acid extract of the seeds of Mucuna pruriens is approximately 3.5 μg/mL. In summary, apart from the results obtained by Kumar et al. (2010) that were markedly high, the values of EC50 from the extracts of Mucuna confirm the presence of important already identified antioxidant compounds in this plant, such as dihydroquercetin, quercetin-3-O-b-D-glucopyranoside and luteolin-8-C-a-L-arabinoside (Du and Li, 2012), and many other as terpenoids, carotenoids and tocopherols to be found yet (Bonis et al., 2010). A further confirmation of the relevant antioxidant role of these compounds emerges from Rajeshwar et al. (2005) who claimed that the extracts of Mucuna pruriens have higher antioxidant activities when compared to different substances, such as BHT.
Table 3 presents the total phenolic content of raw and defatted Mucuna. In agreement with what was observed for antioxidant capacity, the content of total phenols in the different varieties of Mucuna was statistically the same for all the raw samples. The same happens for the meals previously treated at identical conditions of oil extraction (p<0.05).
Table 3. Content of total phenols of raw and defatted Mucuna meal.
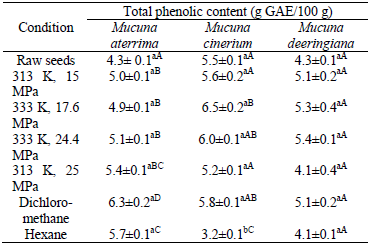
Averages followed by the same lowercase letter in the line and uppercase letters in the column do not differ statistically by Tukey test at p<0.05.
For Mucuna meals defatted by SFE, the phenolic content varied between 4.1 and 6.5 g GAE/100 g of material. However, an overall view of the results shows that neither the temperature nor the pressure have a significant effect (p<0.05) on the concentration of the considered compounds. The same behavior was previously reported at least by two different researchers who examined the total phenolic content in the coffee (Andrade et al., 2012) and in the Agaricus (Mazzutti et al., 2012) by using supercritical CO2. The reason for that is not easily found since it is well-known that the diffusion coefficient and solubility of the phenolic compounds in supercritical fluids tend to increase when increasing the temperature (Eisenmenger et al., 2006; Adil et al., 2008).
At the best operating conditions of oil extraction by SFE in terms of phenolic content, the investigated variable in the defatted sample was approximately 26 % higher than that found in the raw meal of Mucuna aterrima. An analogous comparison for the further examined varieties of Mucuna reveals a similar increase of the content of total phenols in the defatted solid. Such a finding again corroborates the importance of SFE towards the increase of antioxidant power in the meals of Mucuna.
Although the data presented in Table 2 clearly evidence the negligible influence of oil extraction method on antioxidant capacity, a so similar general conclusion can not be stated from the results of total phenolic content in Table 3. For instance, the content of total phenol in the meal of Mucuna aterrima treated with dichloromethane (6.3 ± 0.2 g GAE/100 g) is higher than the highest value of total phenol found in the samples of the same variety defatted by SFE (5.4 ± 0.1 g GAE/100 g). However, such a tendency is not observed for the other two varieties of Mucuna currently investigated. In fact, only two possible generalizations may be made when analyzing the results in Table 3: i) the content of total phenol for Mucuna deeringiana was not statistically different in all the examined samples; ii) except for Mucuna deeringiana, total phenols are present in lower concentration when the meal was preliminarily taken to an operation of oil extraction with hexane (3.2-5.7 g GAE/100 g) instead of dichloromethane (5.8-6.3 g GAE/100 g).
The content of total phenol reported in Table 3 and analogous data found in the literature for Mucuna seeds (≈ 4.3 to 7.8 g/100 g of solid matter) are in the same order of magnitude (Adebowale et al., 2005; Longhi et al., 2011), which validates the experimental results and procedures currently applied. The residual difference found is attributed to the different varieties tested, environmental conditions and agronomic practices during cultivation, as well as to storage at harvest and post-harvest (Vadivel et al., 2011).
Although the literature reports that the presence of polyphenols have a direct impact on the antioxidant activities of Mucuna (Dhanasekaran et al., 2008) and other plant extracts (Elzaawely et al., 2007), the contribution of these species for the overall antioxidant capacity has not been clearly understood. However, the well-established correlation between the low content of total phenols in Table 3 and low antioxidant capacity in Table 2 for the raw seeds, as well as the high content of total phenols in Table 3 and high antioxidant capacity in Table 2 for the defatted seeds, confirms the important role of phenols towards the reduction of free radicals.
IV. CONCLUSIONS
The main aim of the current study was to evaluate the antioxidant capacity and total phenolic content of raw and defatted meals of Mucuna (variety aterrima, cinerium and deeringiana). The determined AC were not statistically different (p<0.05) for defatted meals obtained with supercritical CO2 and organic solvents, which means that the method of oil extraction had a negligible influence on the attempt of increasing the antioxidant capacity of Mucuna meals. No significant differences among the Mucuna varieties in terms of the examined properties were also evidenced. However, the DPPH assay always revealed a practical positive effect of oil removal on the antioxidant capacity. The fall of EC50 observed by reducing the content of oil in the samples confirms the positive role of oil extraction on AC. The highest content of total phenols in the defatted material considering all the methods of oil removal was approximately 18 to 47 % higher than those found in the raw meals when examining samples of the same Mucuna variety. It is a significant additional benefit of oil extraction with final positive impact on AC.
REFERENCES
1. Adebowale, Y.A., I.A. Adeyemi and A.A. Oshodi, "Variability in the physicochemical, nutritional and antinutritional attributes of six Mucuna species," Food Chem., 89, 37-48 (2005).
2. Adil, I.H., M.E. Yener and A. Bayindirh, "Extraction of total phenolics from sour cherry pomace by high pressure solvent and subcritical fluid and determination of the antioxidant activities of the extracts," Separ. Sci. Technol., 43, 1091-1110 (2008).
3. Ambrogi, A., D.A. Cardarelli and R. Eggers, "Separation of natural colorants using a combined high pressure extraction-adsorption process," Latin Am. App. Res., 33, 323-326 (2003).
4. Andrade, K.S., R.T. Gonçalvez, M. Maraschin, R.M. Ribeiro-do-valle, J. Martínez and S.R.S. Ferreira, "Supercritical fluid extraction from spent coffee grounds and coffee husks: Antioxidant activity and effect of operational variables on extract composition," Tal., 88, 544-552 (2012).
5. Batiston, W.P., S.A. Maruyama, S.T.M. Gomes, J.V. Visentainer, N.E. Souza and M. Matsushita, "Total phenolic content and antioxidant capacity of methanolic extracts of ten fruits," Acta Sci. Technol., 35, 581-585 (2013).
6. Benelli, P., C.A.S. Riehlb, A. Smania Junior, E.F.A. Smaniac and S.R.S. Ferreira, "Bioactive extracts of orange (Citrus sinensis L. Osbeck) pomace obtained by SFE and low pressure techniques: Mathematical modeling and extract composition," J. Supercrit. Fluids., 55, 132-141 (2010).
7. Bonis, M.L., A. Tessitore, M.T. Pellecchia, K. Longo, A. Salvatore, A. Russo, D. Ingrosso, V. Zappia, P. Barone, P. Galletti and G. Tedeschi, "Impaired transmethylation potential in Parkinson's disease patients treated with L-Dopa," Neurosci. Lett., 468, 287-291 (2010).
8. Brand-Williams, W., M.E. Cuvelier and C. Berset, "The phenolic constituents of Prunus domestica I. the quantitative analysis of phenolic constituents," Food Scie. Technol., 28, 25-30 (1995).
9. Brunner, G., Gas extraction: an introduction to fundamentals of supercritical fluids and the application to separation processes, Steinkopff, Darmstadt (1994).
10. Cardozo Jr. E.L., L. Cardozo-Filho, O. Ferrarese Filho and E.F. Zanoelo, "Selective liquid CO2 extraction of purine alkaloids in different Ilex paraguariensis progenies grown under environmental influences," J. Agric. Food Chem., 55, 6835-6841 (2007).
11. Corso, M.P., E.A. Silva, M.R.F. Klen, L. Cardozo-Filho, J.N. Santos, L.S. Freitas and C. Dariva, "Extraction of sesame seed (Sesamun indicum L.) oil using compressed propane and supercritical carbon dioxide," J. Supercrit. Fluids., 52, 56-61 (2010).
12. Dhanasekaran, M., B. Tharakan and B.V. Manyam, "Antiparkinson Drug - Mucuna pruriens shows Antioxidant and Metal Chelating Activity," Phytother. Res., 22, 6-11 (2008).
13. Duarte-Almeida, J.M., R.J. Santos, M.I. Genovese and F.M. Lajolo, "Evaluation of the antioxidant activity using the b-carotene/linoleic acid system and the DPPH scavenging method," Food Sci. Technol., 26, 446-452 (2006).
14. Du, Q. and B. Li, "Identification of antioxidant compounds of Mucuna sempervirens by high-speed counter-current chromatographic separation-DPPH radical scavenging detection and their oestrogenic activity," Food Chem., 131, 1181-1186 (2012).
15. Eisenmenger, M., N.T. Dunford, F. Eller, S. Taylor and J. Martinez, "Pilot-scale supercritical carbon dioxide extraction and fractionation of wheat germ oil," J. Am. Oil Chem. Soc., 83, 863-868 (2006).
16. Elzaawely, A.A., T.D. Xuan, H. Koyama and S. Tawata, "Antioxidant activity and contents of essential oil and phenolic compounds in flowers and seeds of Alpinia zerumbet (Pers.) B.L. Burtt. & R. M. Sm," Food Chem., 104, 1648-1653 (2007).
17. Garcia, V.A S., V.F. Cabral, E.F. Zanoelo, C. Silva and L. Cardozo-Filho, "Extraction of Mucuna seed oil using supercritical carbono dioxide to increase the concentration of L-Dopa in the defatted meal," J. Supercrit. Fluids, 69, 75-81 (2012).
18. Garcia, V.AS., C. Silva and L. Cardozo-Filho, "Extraction of mucuna deeringiana seed oil using supercritical carbon dioxide," Acta Scien. Technol., 35, 499-505 (2013).
19. Gelmez, N., S.N. Kincala and M.E. Yener, "Optimization of supercritical carbon dioxide extraction of antioxidants from roasted wheat germ based on yield, total phenolic and tocopherol contents, and antioxidant activities of the extracts," J. Supercrit. Fluids., 48, 270-224 (2007).
20. Hofmann, A.M.S., C. Benincá, V. Kotovicz and E.F. Zanoelo, "Experiments, modeling and control of a dryer-cooler of expanded raw soybean flakes in a hexane extraction plant," J. Am. Oil Chem. Soc., 89, 1929-1938 (2012).
21. Kapoor, I.P.S., B. Singh, G. Singh, C.S. Heluani, M.P. Lampasona and C.A.N. Catalan, "Chemistry and in vitro antioxidant activity of volatile oil and oleoresins of black pepper (Piper nigrum)," J. Agric. Food Chem., 57, 5358-5364 (2009).
22. Kumar, D.S., A.K. Muthu, A.A. Smith and R. Manavalan, "In vitro antioxidant activity of various extracts of whole plant of Mucuna pruriens (Linn)," Inter. J. Pharm. Res., 2, 2063-2070 (2010).
23. Lee, J., N. Koo and D.B. Min, "Reactive oxygen species, aging, and antioxidative nutraceuticals," Compr. Rev. Food Sci. Food Saf., 3, 21-33 (2004).
24. Lemos, C.O.T., V.S.G. Garcia, R.M. Gonçalves, I.C.R. Leal, V.L.D. Siqueira, L. Cardozo-Filho and V.F. Cabral, "Supercritical extraction of neolignans from Piper regnelli var. pallescens," J. Supercrit. Fluids., 71, 64-70 (2012).
25. Longhi, J.G., E. Perez, J.J. Lima and L.M.B. Cândido, "In vitro evalution of Mucuna pruriens (L.) DC. antioxidant activity," Braz. J. Pharm. Sci., 47, 535-544 (2011).
26. Mazzutti, S., R.S. Ferreira, C.A.S. Riehl, A. Smania Jr, F.A. Smania and J. Martínez, "Supercritical fluid extraction of Agaricus brasiliensis: Antioxidant and antimicrobial activities," J. Supercrit. Fluids., 70, 48-56 (2012).
27. Meda, A., C.E. Lamien, M. Romito, J. Millogo and O.G. Nacoulma, "Determination of the total phenolic, flavonoid and proline contents in Burkin Fasan honey, as well as their radical scavenging activity," Food Chem., 91, 571-577 (2005).
28. Mensor, L.L., F.S. Menezes, G.G. Leitão, A.S. Reis, T.C. Santos, C.S. Coube and S.G. Leitão, "Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method," Phytother. Res., 15, 127-130 (2001).
29. Rajeshwar, Y., M. Gupta and U.K. Mazumder, "In vitro lipid peroxidation and antimicrobial activity of Mucuna pruriens seeds," Iran. J. Pharm. Therap., 4, 32-35 (2005).
30. Ruetsch, L., J. Daghero and M. Mattea, "Supercritical extraction of solid matrices. Model formulation and experiments," Latin Am. App. Res., 33, 103-107 (2003).
31. Scherer, R. and H.T. Godoy, "Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem., 112, 654-658 (2009).
32. Shahidi, F. and P.K.J.P.D. Wanasundara, "Phenolic antioxidants," Crit. Rev. Food Sci. Nut., 32, 67-103 (1992).
33. Umoren, U.E., O.O. Effiong, J.C. Onyilagha, E.D. Ekpe and S.O. Okiror, "Chances in nutritional characteristics of the horse-eye bean [Mucuna urens (L.) Medik] subjected to different processing methods," Inter. J. Food Prop., 11, 901-909 (2008).
34. Vadivel, V. and H.K. Biesalski, "Bioactive compounds in velvet bean seeds: effect of certain indigenous processing methods," Inter. J. Food Prop., 15, 1069-1085 (2012).
35. Vadivel, V. and M. Pugalenthi, "Removal of antinutritional/toxic substances and improvement in the protein digestibility of velvet bean seeds during various processing methods," J. Food Sci. Technol., 45, 242-246 (2008).
36. Vadivel, V., W. Stuetz, V. Scherbaum and H.K. Biesalski, "Total free phenolic content and health relevant functionality of Indian wild legume grains: Effect of indigenous processing methods," J. Food Compos. Anal., 24, 935-943 (2011).
37. Yildirim, A., A. Mavi and A.A. Kara, "Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts," J. Agric. Food Chem., 49, 4083-4089 (2001).
38. Zura-Bravo, L., K. Ah-Hen, A. Vega-Gálvez, P. Garcia-Segovia and R. Lemus-Mondaca, "Effect of rehydration temperature on functional properties, antioxidant capacity and structural characteristics of apple (granny smith) slices in relation to mass transfer kinetics," J. Food Proc. Eng., 36, 559-571 (2012).
Received: April 19, 2014.
Accepted: October 7, 2014.
Recommended by Subject Editor: Octavio Furlong












